Free Radical Oxidation of Lipids
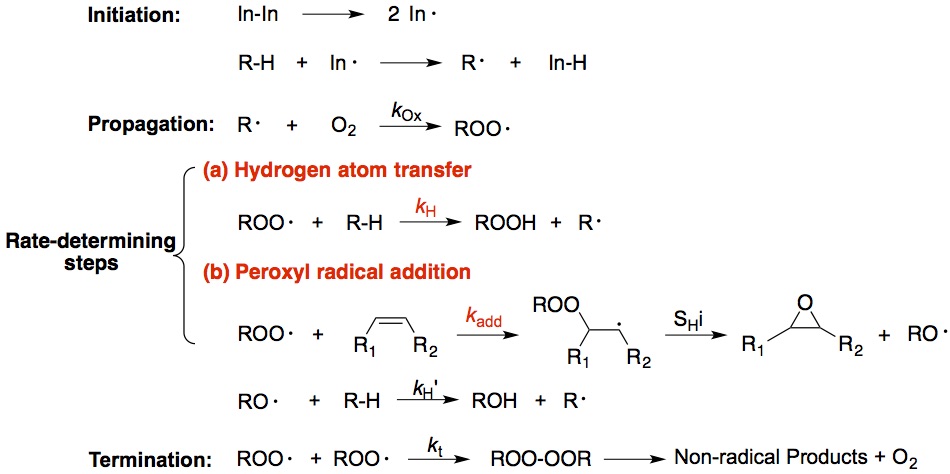
Free radical oxidation proceeds via a chain process that is consisted of three stages: initiation, propagation, and termination [1]. As shown in the Figure below, the rate-determining step in the chain oxidation of lipids is the propagation reaction of the peroxyl radical, where generally two type of processes occur: a) a hydrogen atom is transferred from a donor to the chain-carrying peroxyl radical (hydrogen atom transfer); b) a peroxyl radical adds to a double bond (peroxyl radical addition). In this project, we will use classical “radical clocks” and will develop new “clocks” to measure the rate constants of the both types of rate-determining steps. We aim to understand their reactivities and the formation mechanisms of their products.
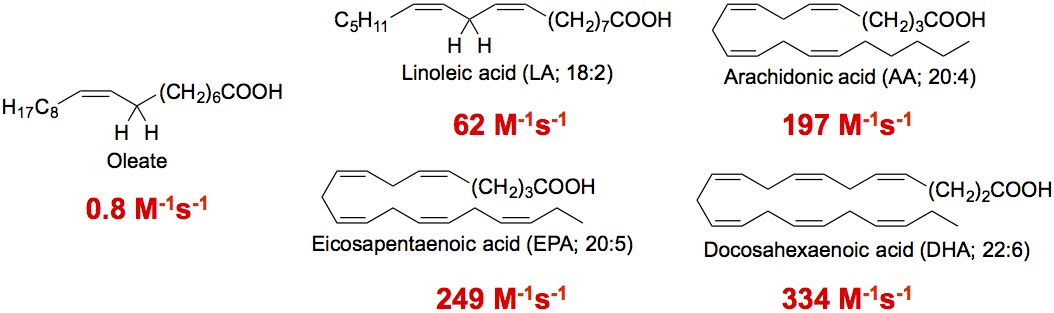
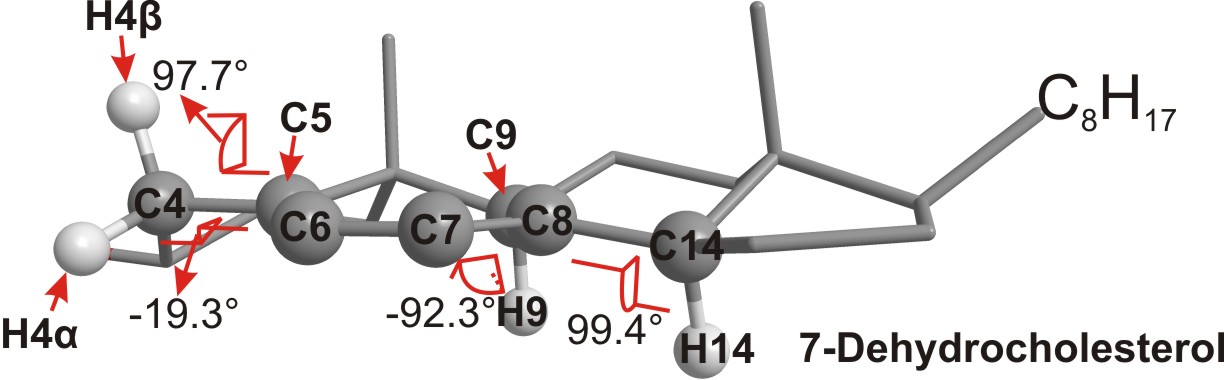
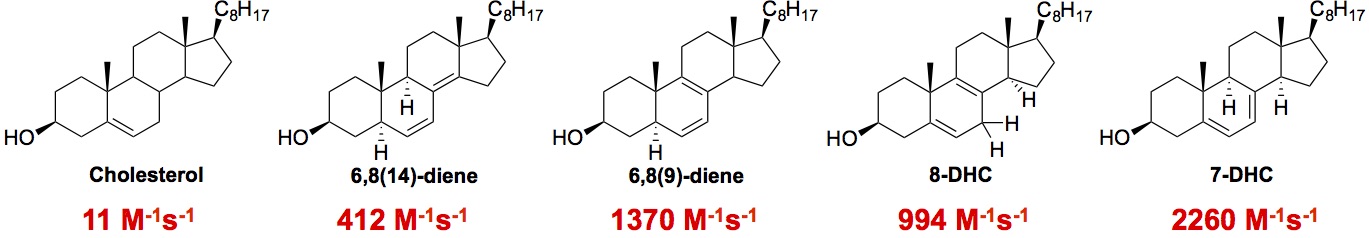
Hydrogen atom transfer. Polyunsaturated fatty acids (PUFAs) are reactive toward hydrogen atom transfer because of the weak C-H at the bis-allylic positions. We recently found that sterols are also excellent hydrogen atom donors, much more reactive than their fatty acid counterparts. We propose that the high reactivities of sterols are due to the substituent pattern on the rings and the well-positioned C-H bonds for removal [2] (see Figure below).
Hydrogen atom transfer rate constants of PUFAs and sterols
3D structure of 7-dehydrocholesterol showing the dihedral angles between the planes containing the allylic C–H bonds (H-9 and H-14) and the adjacent planes containing the double bonds are close to being perpendicular (90º)
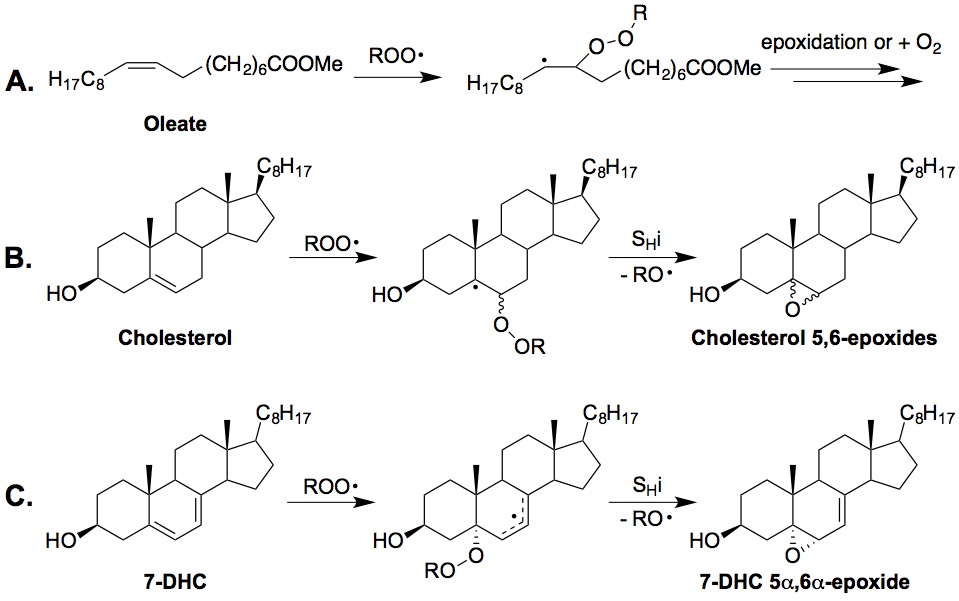
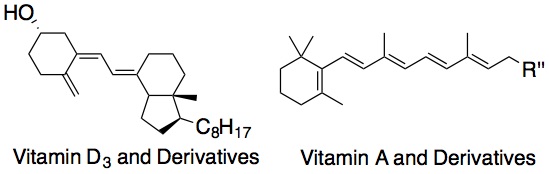
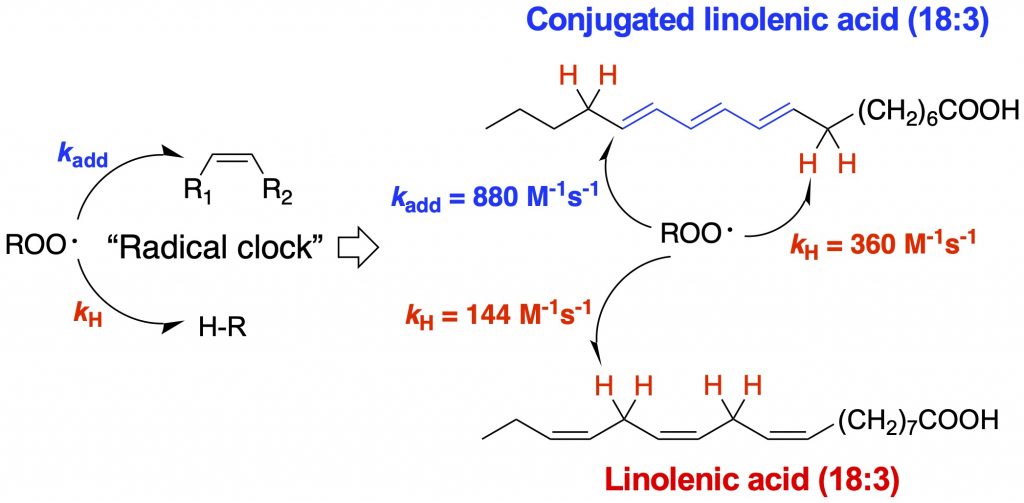
Peroxyl radical addition. The reactivity of a lipid molecule toward this mechanism depends on the stability of the resulting radical. Thus, cholesterol is more reactive than oleate since a more stabilized tertiary radical would be formed (versus a secondary radical). A conjugated molecular system would be prone to peroxyl radical addition since a stabilized allyl radical would be formed upon addition, e.g., 7-dehydrocholesterol. Other conjugated systems that are of particular interest are vitamin D3 and derivatives, as well as vitamin A and derivatives. A collaboration with the Totah lab found that arachidonic acid can also readily undergo peroxyl radical addition reactions, leading to formation of both trans- and cis-epoxyeicosatrienoic acids (EETs) (see Aliwarga et al. Free Radical Biol. Med., 2017). Recently, we developed an improved peroxyl radical clock approach that enabled us to measure the rate constants of peroxyl radical addition reactions (Do. et al., J. Org. Chem., 2021). Using this new approach, we determined the peroxidation rate constants of vitamins A and D, coenzyme Q10, and conjugated polyunsaturated fatty acids for the first time.
Mechanisms of Peroxyl Radical Addition
Conjugated vitamins
Reference
1. Yin, H., Xu, L., and Porter, N. A. (2011) Free Radical Lipid Peroxidation: Mechanisms and Analysis, Chem. Rev. 111, 5944-5972.
2. Xu, L.,* Porter, N. A. (2014) Reactivities and Products of Free Radical Oxidation of Cholestadienols, J. Am. Chem. Soc. 136, 5443−5450.
3. Aliwarga, T.; Raccor, B. S.; Lemaitre, R. N.; Sotoodehnia, N.; Gharib, S. A.; Xu, L.; Totah, R. A. (2017) Enzymatic and free radical formation of cis– and trans– epoxyeicosatrienoic acids in vitro and in vivo. Free Radical Biol. Med. 112, 131-140.
4. Do, Q.; Lee, D. D.; Dinh, A. N.; Seguin, R. P.; Zhang, R.; Xu, L.*, (2021) Development and Application of a Peroxyl Radical Clock Approach for Measuring Both Hydrogen-Atom Transfer and Peroxyl Radical Addition Rate Constants. J. Org. Chem. 86, 153–168.