
Kollman Lab
Research
Kollman Lab
Research
Structure and function of polymerizing enzymes
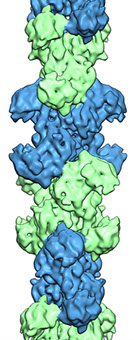
Cellular levels of the nucleotide CTP are tightly regulated, and aberrant CTP production is a hallmark of some cancers. The enzyme CTP synthase (CtpS), which catalyzes the final step in CTP synthesis, is conserved in all organisms, making it an attractive chemotherapeutic and antiparasitic target. Surprisingly, CtpS has recently been shown to form filamentous structures inside cells. Filamentous localization patterns are conserved in bacteria, yeast, Drosophila, and human cells. This striking conservation of filamentous CtpS structures suggests that filament formation plays a functional role in regulating CTP synthesis. Preliminary experiments with recombinant CtpS showed that it forms filaments in vitro (Ingerson-Mahar, et al. 2010). Currently, we are working with purified CtpS and investigating the structure of the filaments it forms using cryo-EM. This work is part of an ongoing collaboration with Zemer Gitai and colleagues at Princeton University. In our recent paper on CtpS (Barry, et al. 2014) Zemer's group showed that polymerization inactivates the enzyme, and we determined the structure by cryo-EM. Ongoing work in our lab aims at understanding the mechanisms of polymerization and inhibition and how whether mechanisms are conserved across species.
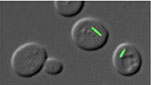
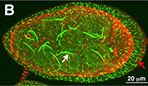
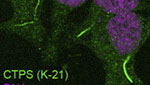
Filamentous localization of CtpS
Caulobacter
S. cerevisiae
Drosophila
HeLa Cells

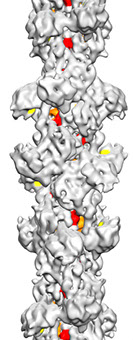
Cryo-EM structure of CtpS
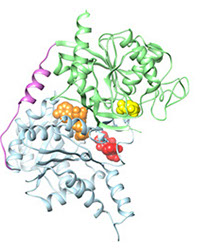
substrate binding sites
CtpS filament structure
CTP bound in the filament
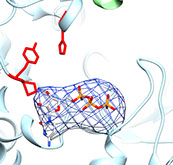
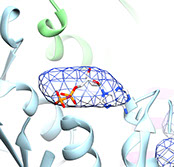
ADP bound in the filament
from Barry, et al. (2014)
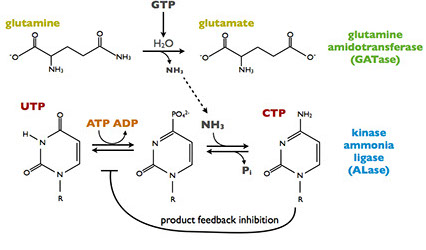
CTP synthesis reaction
CtpS has two domains, each of which catalyzes a different reaction. The kinase ammonia ligase domain catalyzes the conversion of UTP to CTP by replacing a carbonyl an amine group via a phosphorylated intermediate requiring ATP hydrolysis. CtpS activity is partially regulated by CTP product feedback inhibition. Addition of the amine requires ammonia, which is generated by glutamine hydrolysis in the glutamine amidotransferase domain. GTP allosterically activates the glutamine hydrolysis reaction, making CtpS sensitive to the levels of all four ribonucleotides: ATP, CTP, UTP, and GTP.
1959 NE Pacific Street Box 357350
Seattle, WA 98195