
Kollman Lab
Research
Kollman Lab
Research
Plasmid segregation by bacterial actins
Many small plasmids rely on a high copy number, distributed throughout the cell, to ensure at least one copy is maintained by each daughter cell upon division. Many large, low-copy number plasmids, on the other hand, encode active segregation systems to avoid stochastic loss. A surprising variety of partitioning systems exist, but most rely on three components: a centromeric DNA region, a cytomotive filament, and an adaptor protein linking the two. In type II segregation bacterial actin-like protein (ALP) filaments drive plasmid separation. Many large virulence plasmids rely on type II segregation, thus a thorough understanding of the mechanisms of the maintenance of these plasmids will provide opportunities for combating their spread by the targeted disruption of the process.
In the best characterized type II system, ParMRC, ParM filaments are dynamically unstable – ATP binding drives ParM assembly, assembly stimulates ATP hydrolysis which destabilizes the filament, and the filament catastrophically depolymerizes when hydrolysis catches up with the growing end of the filament. This allows multiple ParM filaments to efficiently search the cell for plasmid-adaptor complexes. Adaptor binding stabilizes ParM filaments, allowing processive growth that pushes pairs of plasmids to opposite cell poles, ensuring inheritance in each daughter cell.
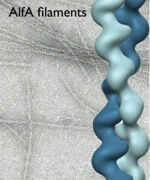
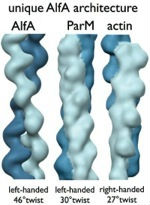
It was initially assumed that all plasmid segregation ALPs would use a ParM-like mechanism. Surprisingly, one ALP, AlfA, has very different dynamic properties from ParM; AlfA is not dynamically unstable, but assembles stably in both ATP and ADP, indicating that it uses a novel mechanism for plasmid maintenance. Our EM structure of individual AlfA filaments showed that its architecture is distinct from both ParM and actin, suggesting a link between structural polymorphisms and variations in dynamic behavior. Single AlfA filaments also form large, apparently well-ordered bundles that were observed to grow by both linear extension and lateral annealing to existing filaments. In cells, AlfA forms stable polymers that often run the full length of the cell; how these structures mediate plasmid segregation remains an open question.
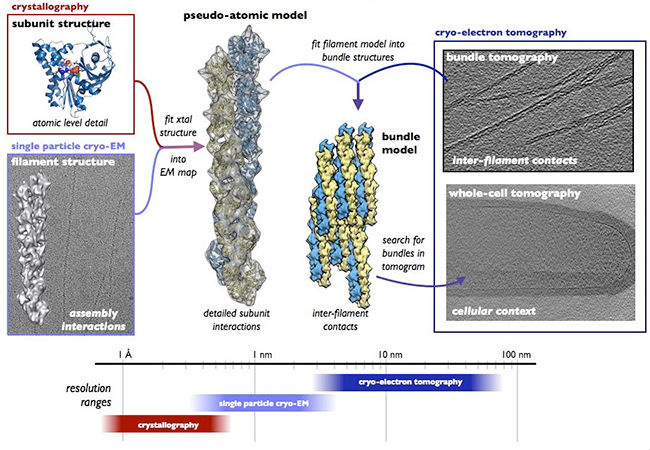
Our current work is focused on elucidating the mechanism of AlfA-based segregation by examining structures of AlfA filaments, filament bundles, and filament-adaptor complexes. Of particular importance are understanding the nature of adaptor binding to AlfA filaments, and whether bundles or single filaments are the physiologically relevant AlfA oligomers. Here, we are combining X-ray crystallography of individual components with cryo-EM reconstructions of the larger structures they form to build atomic resolution models of entire complexes. We are also examining AlfA structure in cells by using correlative light-electron microscopy approaches and cryo-electron tomography.
Overview of our approach to studying AlfA

1959 NE Pacific Street Box 357350
Seattle, WA 98195