 Three stomatal bHLH genes Three stomatal bHLH genes
Similar to many dicot plants, Arabidopsis guard cells differentiate via a series of stereotypical cell divisions, reisterative asymmetric divisions of meristemoids and a single symmetric division of GMC that gives rise to a pair of guard cells. While studies have revealed the presence of cell-cell signals required for proper orientation and density of stomata, genes that direct a series of cell-stete transition, from MMC, meristemoid, GMC, to guard cells, remained elusive. Recent work from our goup and that of Dr. Dominique Bergmann (Stanford) led to a discovery that three closely-related bHLH genes, SPEECHLESS (SPCH), MUTE, and FAMA, direct three key transitional steps leading to stomatal differentiation (Ohashi-Ito and Bergmann, 2006; MacAlister et al., 2007; Pillitteri et al., 2007). Below, we briefly describe about the function of each stomatal bHLH.
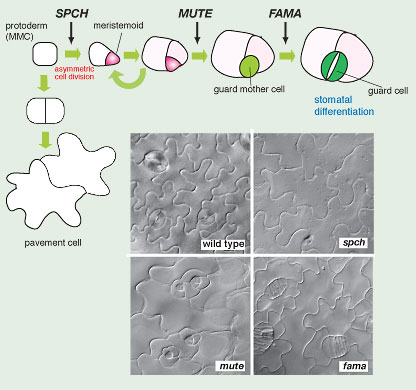
Fig. 6. Sequential actions of three bHLH genes drive three key transitional steps to stomata (copy right, Landes Biosciences, 2007)
Loss-of-function mutants of SPCH or MUTE give rise to aerial organs with no stomata (no "mouth" as implied by their names) (Fig. 6). However, their phenotypes are distinct. Unlike wild type, the epidermis of spch is solely made of jig-saw-puzzle-shaped pavement cells and lacks any stomatal cell lineage (Fig. 6). In contrast, the initial asymmetric division and meristemoid formation occur normally in mute plants. mute meristemoids, however, undergo excessive rounds of asymmetric divisions and subsequently abort instead of differentiating into GMCs. The mute epidermis is characterized by islands of asymmetric divisions that resemble an inward-spiral rosette pattern with an aborted, triangular meristemoid at the center (Fig. 6). The third gene, FAMA (named after the "Goddess of rumor" by D.Bergmann), was previously identified (Ohashi-Ito and Bergmann, 2006). The fama mutation confers reiterative symmetric divisions of GMCs, forming abnormal rows of cells (as if they are "fake mouths") but no mature guard cells (Fig. 6). SPCH, MUTE, and FAMA direct three sequential steps of cell-fate transition during stomatal development: MMC to meristemoid by SPCH, meristemoid to GMC by MUTE, and GMC to guard cells by FAMA (Fig. 6).
SPCH, MUTE, and FAMA encode three most closely-related basic-helix-loop-helix (bHLH) proteins among over 140 bHLH family members in Arabidopsis (MacAlister et al., 2007; Pillitteri et al., 2007; Bailey et al., 2003; Toledo-Ortiz, 2003). This finding highlights a striking parallel between development of stomata and that of specialized cell types in animals, such as muscles and neurons. For example, during skeletal myogenesis, sequential activation of four closely-related myogenic bHLHs are required for skeletal muscle differentiation (Olson, 1990; Weintraub, 1993).
 THREE-STEP RELAY THREE-STEP RELAY
We propose a model by which SPCH, MUTE, and FAMA act sequentially at the key node of transcriptional cascades and together form a 'three-step relay'. In addition to their predicted molecular identities as transcription factors, two lines of evidence support this hypothesis. First, their spaciotemporal expression patterns correlate with each node: SPCH in protodermal cells, MUTE in a subset of meristemoids and early GMCs, and FAMA in GMCs and immature guard cells. The narrow windows of their promoter activities and the fact that no MUTE or FAMA expression was detected in spch mutant plants are highly indicative of the relay.
Second, ectopic overexpression of the bHLHs conferred phenotypes specific to their respective regulatory nodes: SPCH overexpression creating a highly-divided epidermis with increased MMCs, MUTE overexpression conferring an epidermis solely composed of stomata (Fig.7), and FAMA overexpression generating massive clusters of single, unpaired guard cells (Ohashi-Ito and Bergmann, 2006; MacAlister et al., 2007; Pillitteri et al., 2007). While MUTE is required for the transition of a meristemoid to GMC, ectopic MUTE overexpression is sufficient for stomatal differentiation (Fig.7). Therefore, once expressed, MUTE is capable of initiating downstream transcriptional cascades leading to terminal differentiation of stomata, perhaps by passing the 'baton' to FAMA. Consistently, ectopic overexpression of MUTE cannot override the absence of FAMA (our unpublished observation). The final outcome of the relay depends on the strength of key switch gene action. In the weak MUTE overexpressiors, protodermal cells simultaneously execute both pavement- and guard cell gene expression programs. This leads to a formation of hybrid pavement/guard cells, in which a symmetric division plane accompanies a 'faux' pore (Pillitteri et al., 2007). The relay model also explains the single, unpaired guard cells formed by the forced expression of FAMA (Ohashi-Ito and Bergmann, 2006). In this case, the initial cells skip the first two nodes of the relay and begin at the third node, maturation of guard cells. Further experiments will clarify whether the relay is connected directly (whether each bHLH directly upregulates the next player) or indirectly.
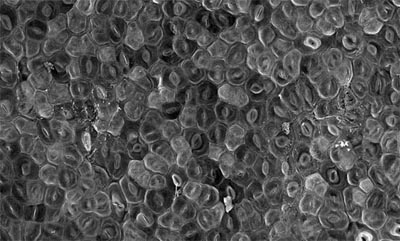
Fig. 7. Constitutive overexpression of MUTE confers an epidermis solely composed of stomata
 SIMILAR YET UNIQUE SIMILAR YET UNIQUE
The distinct overexpression phenotypes by SPCH, MUTE, and FAMA indicate that it is not simply their spaciotemporal expression patterns that specify their biological functions. Consistently, neither MUTE nor FAMA driven by the SPCH promoter rescued the spch mutant phenotype (MacAlister et al., 2007). The uniqueness of their functionality may lie in their amino-acid sequence and structural features. The bHLH domain mediates DNA binding through the basic stretch and homo- and heterodimerization through the HLH domain (Murre et al., 1989). The C-terminal region of SPCH, MUTE, and FAMA shows weak similarity to the ACT domain, a module known for the allosteric regulation of bacterial amino-acid biosynthetic enzymes (Chipman et al., 2001). Recently, the C-terminal ACT-like domain of the Maize R bHLH protein was shown to mediate dimerization (Feller et al., 2006). Formation of homo/heterodimers with specific partners via HLH and ACT-like domains may provide specific functions to these three stomatal bHLH proteins.
 FUTURE PERSPECTIVES FUTURE PERSPECTIVES
Stomatal development offers an excellent system to study cell division, cell-fate specification and cell-type differentiation, all of which lie at the heart of developmental biology. The discovery of SPCH, MUTE, and FAMA as closely-related bHLH proteins provides testable hypotheses to address their function as a molecular switch that drives cell fate changes during stomatal development. Understanding specific domain functions of the stomatal bHLHs and identifying their downstream target genes will establish the molecular basis of the 'three-step relay'. From such studies, one may be able to elucidate the molecular logic of complex cellular behavior supporting physiology and survival of the land plants.
References
Ohashi-Ito K, Bergmann DC (2006) Plant Cell 18, 2493-505.
Pillitteri LJ, Sloan DB, Bogenschutz NL, Torii KU (2007) Nature 445, 501-5.
MacAlister CA, Ohashi-Ito K, Bergmann DC (2007) Nature 445, 537-40.
Bailey PC, Martin C, Toledo-Ortiz G, Quail PH, Huq E, Heim MA, Jakoby M, Werber M, Weisshaar B (2003) Plant Cell 15, 2497-502.
Toledo-Ortiz G, Huq E, Quail PH. (2003) The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell 15, 1749-70.
Weintraub H. (1993) The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell 75, 1241-4.
Olson EN. (1990) MyoD family: a paradigm for development? Genes Dev 4, 1454-61.
Murre C, McCaw PS, Baltimore D. (1989) Cell 56, 777-83.
Chipman DM, Shaanan B. (2001) Curr Opin Struct Biol 11, 694-700.
Feller A, Hernandez JM, Grotewold E. (2006) J Biol Chem 281, 28964-74.
copy right, Keiko U. Torii, 2007 and Landes Biosciences (Plant Signaling and Behavior 2, 311-313. 2007)
|