Baneyx BioNano Laboratories.
|
|
..Research
|
|
|
|
|
|
|
|
|
|
|
|
|
| .Overview |
| Research in the Baneyx laboratory intersects engineering, biology and nanotechnology. How proteins fold into intricate three-dimensional shapes - or why they sometimes fail to do so - has profound implications in medicine and biotechnology. We study the genetics, regulation and structure-function relationship of molecular chaperones, a class of proteins that help other polypeptides reach a correct conformation. We exploit this fundamental knowledge to facilitate the production of bioactive recombinant proteins in the bacterium Escherichia coli, to build biosensors, and to explore the connection between protein (mis)folding and neurodegenerative diseases. |
|
|
|
|
|
| In the nanobiotechnology arena, we use combinatorial techniques to isolate and evolve short peptides binding to inorganic or synthetic materials. We bring to bear a variety of experimental and theoretical approaches to better understand the mechanisms of solid adhesion. We engineer solid-binding peptides within well-characterized protein "scaffolds" and use the resulting designer proteins to nucleate, organize and assemble nanostructured materials, with the aim of building systems and devices exhibiting superior mechanical or opto-electronic properties. |
|
|
 |
|
| Colorized SEM image of Escherichia coli cells. E. coli is a well characterized organism suitable for the high-level expression of recombinant proteins. |
|
|
|
| .Protein Folding, Molecular Chaperones and Protein Expression |
| In order to display biological activity, newly synthesized proteins must fold into a precise three-dimensional conformation stabilized by hydrogen bonds, van der Waals interactions, hydrophobic interactions, salt bridges and disulfide bonds. Although all the information necessary for a protein to reach a proper structure is contained in its amino acid sequence, recombinant proteins of therapeutic or commercial interest are often unable to properly fold when expressed at high levels in bacteria or higher cells. The resulting misfolded proteins may either be degraded by cellular proteases or, more commonly, become sequestered into insoluble - and usually inactive - proteinaceous aggregates known as inclusion bodies. Protein misfolding and aggregation is also associated with a number of neurodegenerative and degenerative disorders including Parkinson's, Alzheimer's, Huntington's, Creutzfeld-Jakob and Gaucher's diseases.
Molecular chaperones are a class of proteins that help other polypeptides reach a proper conformation or cellular location. An improved understanding of the physiological role and mechanism of action of molecular chaperones, and of how they cooperate with each other in the management of protein folding/misfolding, has implications in the high-yield low-cost production of recombinant proteins, in uncovering the etiology of certain human diseases, and in developing therapeutic treatments for these disorders.
We study the function, biochemistry, regulation and practical applications of molecular chaperones in heterologous protein production. To read publications from our laboratory on this topic, clik here. To learn more about E. coli molecular chaperones, click on your favorite chaperone below or follow this link.
|
|
|
|
|
|
|
|
|
 |
|
|
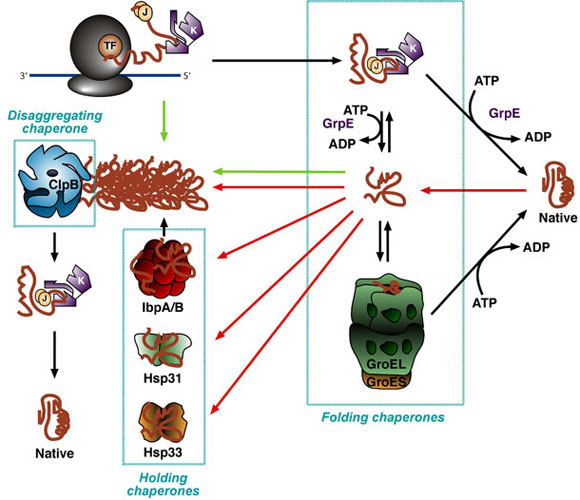
| The molecular chaperone network in the cytoplasm of E. coli. Nascent proteins requiring the assistance of molecular chaperones to reach a proper conformation first encounter ribosome-bound trigger factor (TF) or the DnaK-DnaJ system (KJ). Both chaperones bind solvent-exposed stretches of hydrophobic amino acids in partially folded substrate proteins, shielding them for the solvent and from each other, and precluding their aggregation. Following undocking from TF or GrpE-mediated release from DnaK, folding intermediates may reach a native conformation, cycle back to DnaK-DnaJ or be transferred to the central chamber of GroEL for folding at infinite dilution upon GroES capping. In times of stress (e.g., heat, acid shock or oxidative conditions), a subset of cellular proteins unfolds and aggregates (red arrows). The small heat shock proteins IbpA/B bind partially folded species on their surface to serve as a reservoir of folding intermediate until stress abates and folding chaperones (DnaK-DnaJ and GroEL-GroES) become available for their refolding. IbpA/B also intercalate within large protein aggregates to facilitate their dissolution and the reactivation by the disaggregase ClpB and the DnaK-DnaJ system. The holding chaperone Hsp33 becomes important under oxidative stress conditions, when reactive oxygen species damage cellular proteins and cause them to misfold. Hsp31 stabilizes unfolding intermediates under times of intense cellular stress and following growth medium acidification. Recombinant proteins that miss an early interaction with TF or DnaK-DnaJ, that undergo multiple cycles of abortive interactions with folding chaperones, or that titrate them out, accumulate within inclusion bodies (green arrows). |
|
|
|
|
|
| .Cellular Stress and Sensor Development |
|
|
|
|
| In the wild, microorganisms are continuously exposed to stressful conditions including temperature upshifts and downshifts, abundance or dearth of nutrients, and changes in the physico-chemical composition of the growth environment. To survive these fluctuations, cells have evolved remarkable adaptive mechanisms which typically involve a transient increase in the synthesis of protective proteins (including molecular chaperones) that are well equiped to handle stress. We are interested in gaining a global understanding of stress responses in E. coli and their implications on cell physiology, recombinant protein expression, and genetic redundancy. On a more pragmatic front, we are exploiting stress responses to search for new antibacterial agents and to develop whole-cell sensors that provide a chromogenic or optical response in the presence of target analytes. Follow this link for relevant reviews and publications from our laboratory. |
|
 |
|
|
| Emission wavelength-shifted mutants of firefly luciferase. Firefly (P. pyralis) luciferase produces yellow-green light upon oxidation of D-luciferin in the presence of ATP and molecular oxygen. Using error-prone PCR, we have isolated mutant enzymes producing orange and red light and are exploring their applications in sensor design and opto-electronics. |
|
|
|
|
|
|
|
| Nanotechnology can be broadly defined as the exploration and exploitation of unique phenomena occurring at the atomic, molecular and supra-molecular scales to create materials, devices and systems exhibiting novel properties and functions. This emerging field has the potential to radically alter the way we build composite materials, design electronic, optical and magnetic devices, perform catalysis, study biology, and diagnose and treat human diseases. Nanomaterials displaying useful properties as a result of their size, composition and/or architecture are already finding their way to the clinic (e.g., quantum dots, noble metal nanoparticle and nanoshells, paramagnetic nanoparticles and carbon nanotubes) and their penetration in other sectors is accelerating. Yet, most nanomaterials are difficult and expensive to synthetize and traditional approaches do not have the flexibility and versatility that is needed for the construction of the active nanostructures and systems of tomorrow. |
|
|
 |
|
| Protein-aided nanofabrication. A designer protein was used to nucleate ≈2 nm diameter particles of cuprous oxide (dark spots) and the resulting protein shell-cuprous oxide core nanoparticles were assembled onto a DNA circle by taking advantage of the built-in DNA binding activity of the designer protein. |
|
|
| In nature, proteins are the ultimate conductors of material synthesis, orchestrating the nucleation, growth and assembly of a variety of soft and hard tissues with precise control of phase composition and architecture from the nano to the mesoscales. Although natural biomineralizing proteins only work on a limited number of compositions, it is now possible to use combinatorial techniques to select small peptides binding to solid phases of engineering interest. Using standard molecular biology techniques, these peptides can be fused within the framework of other proteins to generate "designer proteins" that combine the solid-binding (and sometimes solid-synthesizing) ability of the guest peptide with the original properties of the host scaffold (e.g., self-assembly, ligand binding, catalysis, light emission...) As part of the Genetically Engineered Materials Science & Engineering Center (an NSF MRSEC), and in collaboration with the Schwartz laboratory, we are building and characterizing designer proteins that will be suitable for the biofabrication of functional hybrid nanomaterials with programmed composition, phase, and topology. We also use eubacterial and archaebacterial S-layer proteins, which have the ability to self-assemble into nearly all space group symmetries, to template the growth of inorganic nanostructures into precise patterns. The resulting surfaces may be used for the construction of highly sensitive sensors and have applications in catalysis and opto-electronic devices construction. Follow this link for relevant reviews and publications from the Baneyx lab. |
|
|
|
 |
|
 |
|
|
| Templated surfaces. The surface layer (S-layer) protein of S. ureae assembles in a square crystalline structure that can be used as a resist to template the growth of inorganic materials (bottom). Images have been colorized. |
|
|
SSelected Publications: Folding & Chaperones
|
|
Reviews
|
| Baneyx, F. and M. Mujacic. 2004. "Recombinant protein folding and misfolding in Escherichia coli". Nat. Biotechnol. 22:1399-1408. |
| Baneyx, F and J.L Palumbo. 2003. "Improving heterologous protein folding via molecular chaperone and foldase co-expression". Methods Mol. Biol. 205:171-197. |
| Baneyx, F. 1999. "Recombinant protein expression in Escherichia coli". Curr. Opin. Biotechnol. 10:411-421. |
| Thomas, J.G., A. Ayling and F. Baneyx. 1997. "Molecular chaperones, folding catalysts and the recovery of biologically active recombinant proteins from E. coli: to fold or to refold". Appl. Biochem. Biotech. 66:197-238. |
| Hsp31 |
| Mujacic, M. and F. Baneyx. 2007. "Chaperone Hsp31 contributes to acid resistance in stationary phase Escherichia coli". Appl. Environ. Microbiol. 73:1014-1018. |
| Mujacic, M. and F. Baneyx. 2006. "Regulation of Escherichia coli hchA, a stress-inducible gene encoding molecular chaperone Hsp31". Mol. Microbiol. 60:1576-1589. |
| Sastry, M.S.R., P. Quigley, W.G.J. Hol and F. Baneyx. 2004. "The linker-loop region of E. coli chaperone Hsp31 functions as a thermal gate that modulates high affinity substrate binding at elevated temperatures". Proc. Natl. Acad. Sci. USA 101:8587-8592. |
| Mujacic, M., M. Bader and F. Baneyx. 2004. "Escherichia coli Hsp31 functions as a holding chaperone that cooperates with the DnaK-DnaJ-GrpE system in the management of protein misfolding under severe stress conditions". Mol. Microbiol. 51:849-859. |
| Quigley, P., K. Korotkov, F. Baneyx and W.G.J. Hol. 2004. "A new native EcHsp31 structure suggests key role of structural flexibility for chaperone function". Protein Sci. 13:269-277. |
| Quigley, P., K. Korotkov, F. Baneyx and W.G.J. Hol. 2003. "The 1.6 Å crystal structure of the class of chaperones represented by E. coli Hsp31 reveals a putative catalytic triad". Proc. Natl. Acad. Sci. USA 100:3137-3142. |
| Sastry, M.S.R., K. Korotkov, Y. Brodsky and F. Baneyx. 2002. "Hsp31, the Escherichia coli yedU gene product, is a molecular chaperone whose activity is inhibited by ATP at high temperatures". J. Biol. Chem. 277:46026-46034. |
| ClpB |
| Chow, I.-T., M.E. Barnett, M. Zolkiewski and F. Baneyx. 2005. "The N-terminal domain of Escherichia coli ClpB enhances chaperone function". FEBS Lett. 579:4242-4248. |
| Chow, I.-T.and F. Baneyx. 2005. "Coordinated synthesis of the two ClpB isoforms improves the ability of Escherichia coli to survive thermal stress". FEBS Lett. 579:4235-4241. |
| Thomas, J.G and F. Baneyx. 2000. "ClpB and HtpG facilitate de novo protein folding in stressed Escherichia coli cells". Mol. Microbiol. 36:1360-1370. |
| IbpA/B |
| Shearstone, J.R. and F. Baneyx. 1999. "Biochemical characterization of the small heat shock protein IbpB from Escherichia coli". J. Biol. Chem. 274:9937-9945. |
| Thomas, J.G. and F. Baneyx. 1998. "Roles of the Escherichia coli small heat shock proteins IbpA and IbpB in thermal stress management: comparison with ClpA, ClpB, and HtpG in vivo". J. Bacteriol. 180:5165-5172. |
| DnaK-DnaJ-GrpE and GroEL-GroES |
| Amatore, D. and F. Baneyx. 2003. "Insertion mutagenesis of Escherichia coli GroEL". Biochem. Biophys. Res. Commun. 302:246-252. |
| Thomas, J.G. and F. Baneyx. 1997. "Divergent effects of chaperone overexpression and ethanol supplementation on inclusion body formation in recombinant Escherichia coli". Protein Express. Purif. 11:289-296. |
| Schneider, E.L., J.G. Thomas, J.A. Bassuk, E.H. Sage and F. Baneyx. 1997. "Manipulating the aggregation and oxidation of human SPARC in the cytoplasm of Escherichia coli". Nat. Biotechnol. 15:581-585. |
| Thomas, J.G. and F. Baneyx. 1996. "Protein folding in the cytoplasm of Escherichia coli: requirements for the DnaK-DnaJ-GrpE and GroEL-GroES molecular chaperone machines". Mol. Microbiol. 21:1185-1196. |
| Thomas, J.G. and F. Baneyx. 1996. "Protein misfolding and inclusion body formation in Escherichia coli.cells overexpressing heat shock proteins". J. Biol. Chem. 271:11141-11147. |
| Ayling, A. and F. Baneyx. 1996. "Influence of the GroE molecular chaperone machine on the in vitro refolding of Escherichia coli beta-galactosidase". Protein Sci. 5:478-487. |
| Baneyx, F. and A.A. Gatenby. 1992. "A Mutation in GroEL interferes with protein folding by reducing the rate of discharge of sequestered polypeptides". J. Biol. Chem. 267:11637-11644. |
| TOP |
|
|
|
Selected Publications: Stress and Sensing
|
| Reviews |
| Baneyx, F and M. Mujacic. 2003. "Cold-inducible promoters for heterologous protein expression". Methods Mol. Biol. 205:1-18. |
| Sensing |
| Shapiro, E. and F. Baneyx. 2007. "Stress-activated bioluminescent E. coli sensors for antimicrobial agents detection". J. Biotechnol. 132:487-493. |
| Shapiro, E., C. Lu and F. Baneyx. 2005. "A set of multicolored Photinus pyralis luciferase mutants for in vivo bioluminescence applications". Protein Eng. Des. Sel. 18:581-587. |
| Shapiro, E and F. Baneyx. 2002. "Stress-based identification and classification of antibacterial agents: second generation Escherichia coli reporter strains and optimization of detection". Antimicrob. Agents Chemother. 46:2490-2497. |
| Bianchi, A.D. and F. Baneyx. 1999. "Stress responses as a tool to detect and characterize the mechanism of action of antibacterial agents". Appl. Environ. Microbiol. 65:5023-5027. |
| Cold shock |
| Mujacic, M., Cooper, K.W. and F. Baneyx. 1999. "Cold-inducible cloning vectors for low temperature protein expression in Escherichia coli: application to the production of a toxic and proteolytically sensitive fusion protein". Gene 238:325-332. |
| Vasina, J.A., M.S. Peterson and F. Baneyx. 1998. "Scale-up and optimization of the low-temperature-inducible cspA promoter system". Biotechnol. Prog. 14:714-721. |
| Vasina, J.A. and F. Baneyx. 1997. "Expression of aggregation-prone recombinant proteins at low temperatures: a comparative study of the E. coli cspA and tac promoter systems". Protein Express. Purif. 9:211-218. |
| Vasina, J.A. and F. Baneyx. 1996. "Recombinant protein expression at low temperatures under the transcriptional control of the major E. coli cold shock promoter cspA". Appl. Environ. Microbiol. 62:1444-1447. |
| Other stresses |
| Mujacic, M. and F. Baneyx. 2007. "Chaperone Hsp31 contributes to acid resistance in stationary phase Escherichia coli". Appl. Environ. Microbiol. 73:1014-1018. |
| Bianchi, A.D. and F. Baneyx. 1999. "Hyperosmotic shock induces the sigma32 and sigmaE stress regulons of Escherichia coli". Mol. Microbiol. 34:1029-1038. |
| Thomas, J.G. and F. Baneyx. 1996. "Influence of a global deregulation of the heat-shock response on the expression of aggregation-prone proteins in Escherichia coli". Ann. New York Acad. Sci. 782:478-485. |
| TOP |
|
|
|
Selected Publications: Bionanotechnology
|
| Reviews |
| Baneyx, F. and D.T. Schwartz. 2007. "Selection and analysis of solid-binding peptides". Curr. Opin. Biotechnol. 18:312-317. |
| Sarikaya, M., C. Tamerler, D.T. Schwartz, and F. Baneyx. 2004. "Materials assembly and formation using engineered polypeptides". Annu. Rev. Mat. Res. 34:373-408. |
| Sarikaya, M., C. Tamerler, A.K.-Y. Jen, K. Schulten and F. Baneyx. 2003. "Molecular biomimetics: nanotechnology through biology". Nat. Mater. 2:577-585. |
| Designer proteins |
| Sengupta, A., C.K. Thai, M.S.R. Sastry, J.F. Matthaei, D.T. Schwartz, E.J. Davis and F. Baneyx. 2008. "A genetic approach for controlling the binding and orientation of proteins on nanoparticles". Langmuir 24:2000-2008. |
| Choe, W.-S., M.S.R. Sastry, C.K. Thai, H. Dai, D.T. Schwartz and F. Baneyx. 2007. "Conformational control of inorganic adhesion in a designer protein engineered for cuprous oxide binding". Langmuir. 23:11347-11350. |
| Dai, H., W.-S. Choe, C.K. Thai, M. Sarikaya, B. Traxler, F. Baneyx and D.T. Schwartz. 2005. "Nonequilibrium synthesis and assembly of hybrid inorganic-protein nanostructures using an engineered DNA-binding protein" J. Am. Chem. Soc. 127:15637-15643. |
| Thai, C.K., H. Dai, M.S.R. Sastry, M. Sarikaya, D.T. Schwartz and F. Baneyx. 2004. "Identification and characterization of Cu2O and ZnO binding polypeptides by Escherichia coli cell surface display: towards an understanding of metal oxide binding". Biotechnol. Bioeng. 87:129-137. |
| Dai, H., C.K. Thai, M.Sarikaya, F. Baneyx, and D. T. Schwartz. 2004. "Through-mask anodic patterning of copper surfaces and film stability in biological media". Langmuir 20:3483-3486. |
| S-layer proteins |
| Allred, D.B., A. Cheng, M. Sarikaya, F. Baneyx and D.T. Schwartz. 2008. "3-D architecture of inorganic nanoarrays electrodeposited through an S-layer protein mask". Nano Lett. 8:1434-1438. |
| Allred, D.B., M. Sarikaya, F. Baneyx and D.T. Schwartz. 2007. "Bacterial surface-layer proteins for electrochemical nanofabrication". Electrochim. Acta. 53:193-199. |
| Presenda, A., D.B. Allred, D.B., F. Baneyx, D.T. Schwartz and M. Sarikaya. 2007. "Stability of S-layer proteins for electrochemical nanofabrications". Colloids Surf. B. 57:256-261. |
| Allred, D.B., M.T. Zin, H. Ma, M. Sarikaya, F. Baneyx, A.K.-Y. Jen and D.T. Schwartz. 2007. "Direct nanofabrication and TEM analysis on a suite of easy-to-prepare ultrathin film substrates". Thin Solid Films 515:5341-5347. |
| Allred, D.B., M. Sarikaya, F. Baneyx and D.T. Schwartz. 2005. "Electrochemical nanofabrication using crystalline protein masks". Nano Lett. 5:609-613. |
| TOP |
|
|
| | home | research | publications | people | photos | links | teaching | intranet |
Contact: François Baneyx, University of Washington, Department of Chemical Engineering, Box 351750, Seattle, WA Tel: 206-685-7659 Fax: 206-685-3451 E-mail: baneyx@uw.edu
© 2008-2009 François Baneyx - All Rights Reserved
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|











