|
John D. Scott
|
John Scott is interested in the specificity of signal transduction events that are controlled by anchoring proteins, which facilitate rapid signal transduction by optimally positioning protein kinases and phosphatases in the vicinity of their activating signals and close to their substrates.
Research Summary
Cells respond to environmental cues by mobilizing signal transduction enzymes. Spatial organization provided by scaffolding proteins guides this flow of molecular information to particular subcellular sites. We study A-kinase anchoring proteins (AKAPs) that tether the cAMP-dependent protein kinase (PKA), other protein kinases, and protein phosphatases to control the phosphorylation status of substrates. We Initially discovered AKAPs as simple binding proteins purported to direct PKA to specific locations. However, we now appreciate that this functionally related but structurally diverse group of proteins comprise a large family of multivalent enzyme scaffolds that synchronize many complex cellular events. They constrain broad-specificity enzymes in customized macromolecular units to enable cells to respond efficiently and accurately to the ebb and flow of diffusible second-messenger signals. Our most recent discovery has been that that active enzymes have a restricted range of motion within AKAP assemblies. This ‘signaling island’ concept radically changes our view of how AKAP complexes operate and indicates that protein phosphorylation is much more regionally confined than previously appreciated. Another important concept emerging from these discoveries is that AKAP-enzyme interfaces are potential targets for the disruption of pathological signaling. The lab is now focused on exploring and exploiting the pharmacological modulation of kinases and phosphatases within the confines of AKAP nano-compartments.
Model of a cell showing the location of several AKAP signaling complexes.
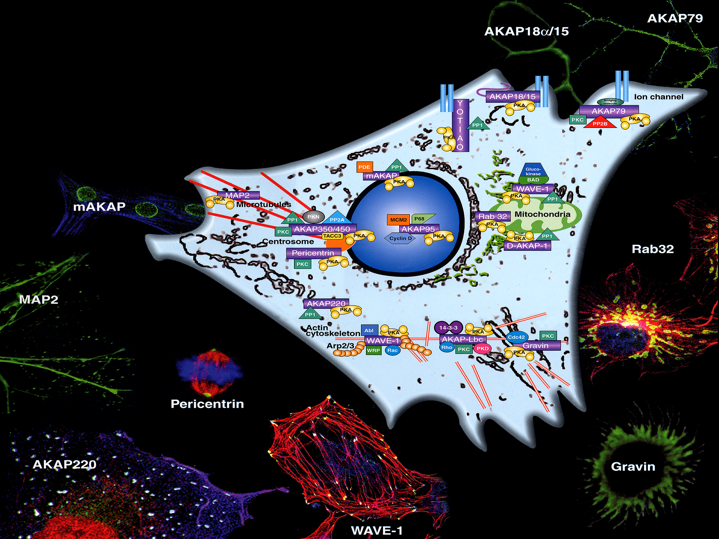
click image to enlarge