1. Cancer Therapy
Over 18 million people in the U.S. are currently battling cancer. Cytotoxic chemotherapy remains the preferred frontline strategy used against most types of cancer. While effective, treatment-related side effects such as major organ damage, infertility, immunosuppression, and nausea/vomiting severely compromise patient quality of life. We are developing technologies for improved detection and treatment of cancer.
Ongoing projects in this area include:
- Synthetic materials for adoptive T cell therapy. T cell immunotherapy demonstrates remarkable anti-cancer activity in the clinic. We are working to develop materials that improve the manufacturing and in vivo performance of T cell therapies.
- Polymers for drug delivery. With Dr. Patrick Stayton, we are synthesizing polymeric drug carriers to change the biodistribution of small molecule drugs for immunotherapy, thereby reducing toxicity.
- Cancer vaccine delivery. Cancer vaccines are a promising inmuno-oncology approach that can elicit anti-cancer protection from the immune system. Together with Dr. Patrick Stayton and Dr. Nora Disis, we are developing polymer-based carriers that can be recognized by and activate dendritic cells for antigen presentation, leading to cancer prevention and therapy.

Selected recent publications:
- Heinze, C.M.*, Pichon, T.J.*, Wu, A.Y., Baldwin, M., Matthaei, J., Song, K., Sylvestre, M., Gustafson, J., White, N.J., Jensen, M.C. and Pun, S.H. Spatial Control of CAR T Cell Activation Using Tumor-Homing Polymers. Journal of the American Chemical Society 2025, v147(6), 5149-5161.
- Nguyen, D.C.*, Song, K.*, Jokonya, S., Yazdani, O., Sellers, D.L., Wang, Y., Zakaria, A., Pun, S.H., and Stayton, P.S. Mannosylated STING agonist ‘drugamers’ for dendritic cell-mediated cancer immunotherapy. ACS Central Science 2024, 10(3), 666–675.
- Olshefsky, A., Benasutti, H., Sylvestre, M., Butterfield, G.L., Rocklin, G.J., Richardson, C., Hicks, D.R., Lajoie, M.J., Song, K., Leaf, E., Treichel, C., Decarreau, J., Ke, S., Kher, G., Carter, L., Chamberlain, J.S., Baker, D., King, N.P., and Pun, S.H. In vivo selection of synthetic nucleocapsids for tissue targeting. PNAS 2023, 120(46), e2306129120.
- Lv, S.*, Song, K.*, Yen, A., Peeler, D.J., Nguyen, D.C., Olshefsky, A., Sylvestre, M., Srinivasan, S., Stayton, P.S., and Pun, S.H. Well-defined mannosylated polymer for peptide vaccine delivery with enhanced antitumor immunity. Advanced Healthcare Materials 2022, 11(9), 2101651.
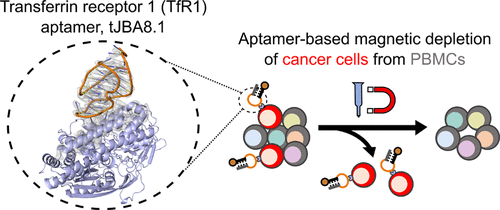
- Kacherovsky, N.*, Cardle, I.I.*, Cheng, E.L., Yu, J.L., Baldwin, M.L., Salipante, S.J., Jensen, M.C., and Pun, S.H. Traceless aptamer-mediated isolation of CD8+ T cells for chimeric antigen receptor T-cell therapy. Nature Biomedical Engineering 2019, 3, 783-795.
We are grateful to the following current and past funding sources: NIH NCI, NIH NIBIB, NSF DMR, Alliance for Cancer Gene Therapy, Washington Research Foundation.