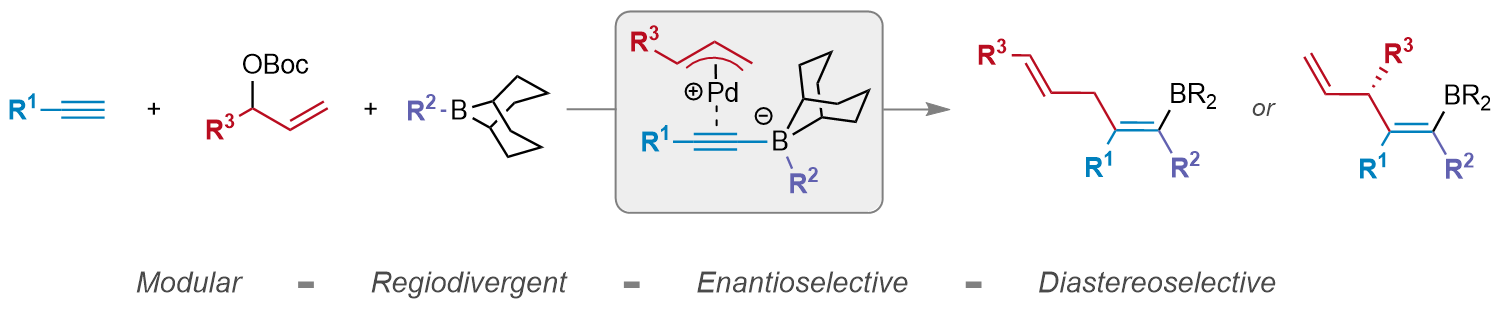
| 51. Yang, L.; Detels, M. C.; Lalic, G. Enantioselective Trifunctionalization of Terminal Alkynes J. Am. Chem. Soc. ASAP |link| |
|
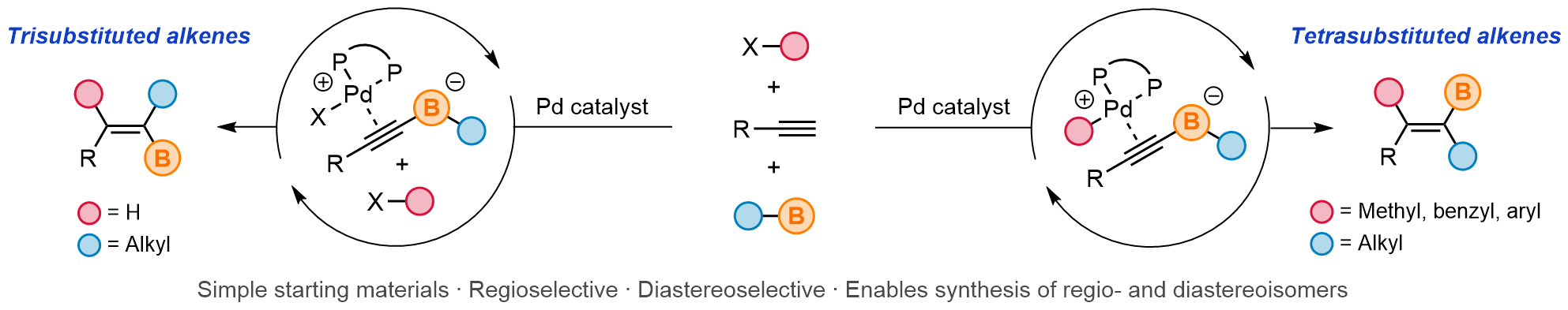
| 50. Gardner, B. W.; Chung, C. P.; Pu, M. R.; Lalic, G. Synthesis of Highly Substituted Alkenes from Terminal Alkynes J. Am. Chem. Soc. 2025, 147, 33177-33184. |link| |
|
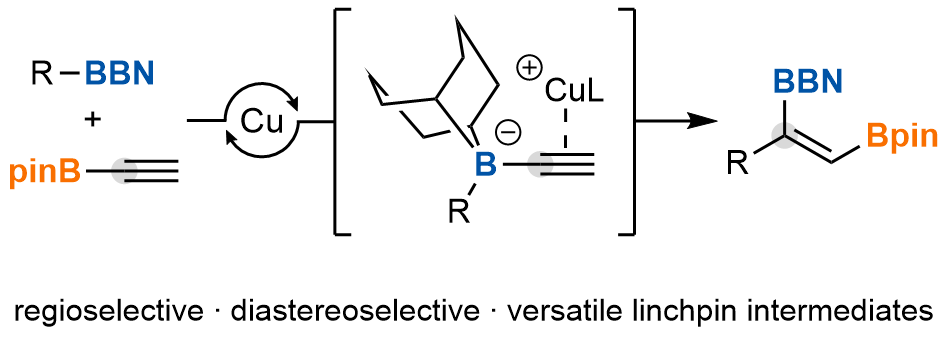
| 49. Gardner, B. W.; Lalic, G. Catalytic C(sp2)-Insertive Homologation of Alkylboranes Nat. Chem. 2025, 17, 1418-1424. |link| |
|
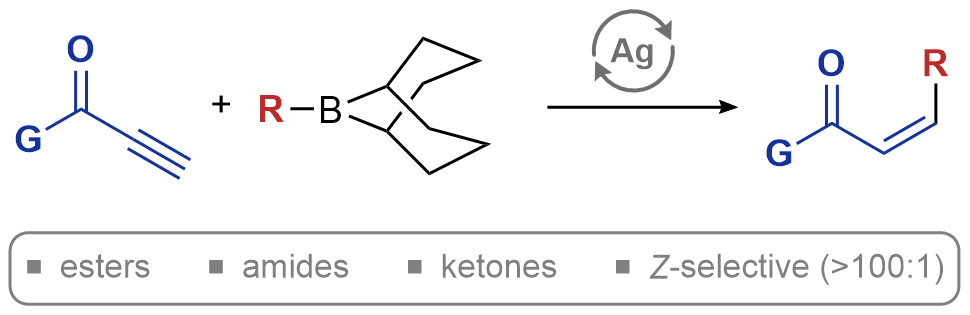
| 48. Shaff, A. B.; Hazra, A.; Gardner, B. W.; Lalic, G. Selective Synthesis of Z-Michael Acceptors via Hydroalkylation of Conjugated Alkynes J. Am. Chem. Soc. 2025, 147, 27-32. |link| |
|
| 47. Yang. L.; Lalic, G. Regio- and Diastereoselective Synthesis of Trisubstituted Alkenes Through Hydroalkylation of Alkynyl Boronamides Angew. Chem. Int. Ed. 2024, e202409429. |link| |

|
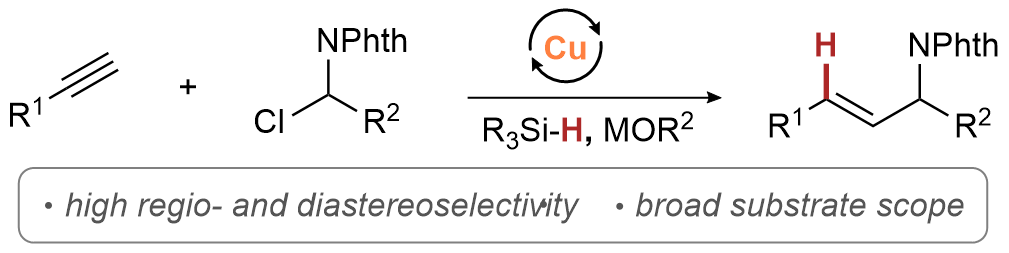
| 46. Wang, B.; Hazra, A.; Lalic, G. Regio- and Diastereoselective Synthesis of E-Allylic Amines through Hydroalkylation of Terminal Alkynes ACS Catal. 2024, 14, 6021−6027. |link| |
|
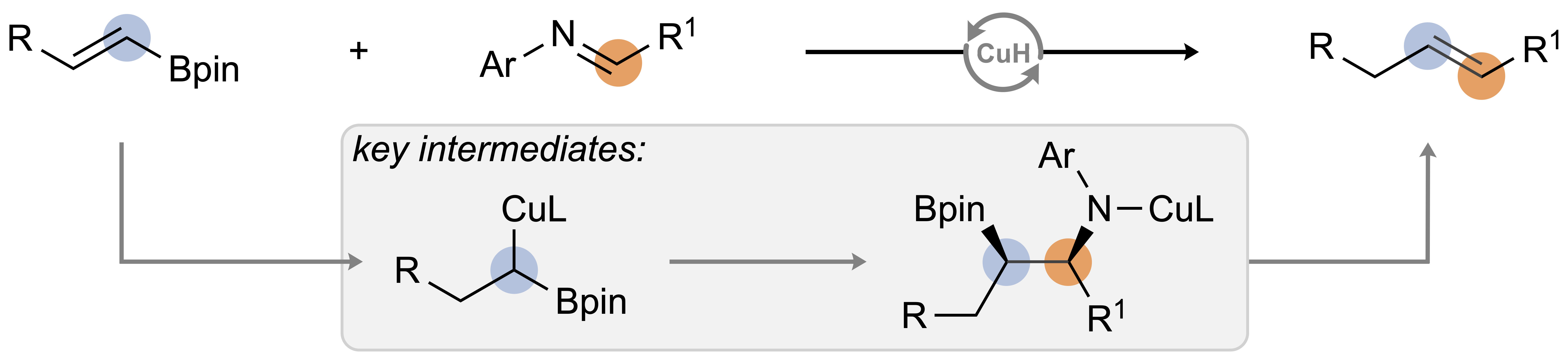
| 45. Baumann, J. E.; Chung, C. P.; Lalic, G. Stereoselective Copper-Catalyzed Olefination of Imines Angew. Chem. Int. Ed. 2023, early view, e202316521. |link| |
|
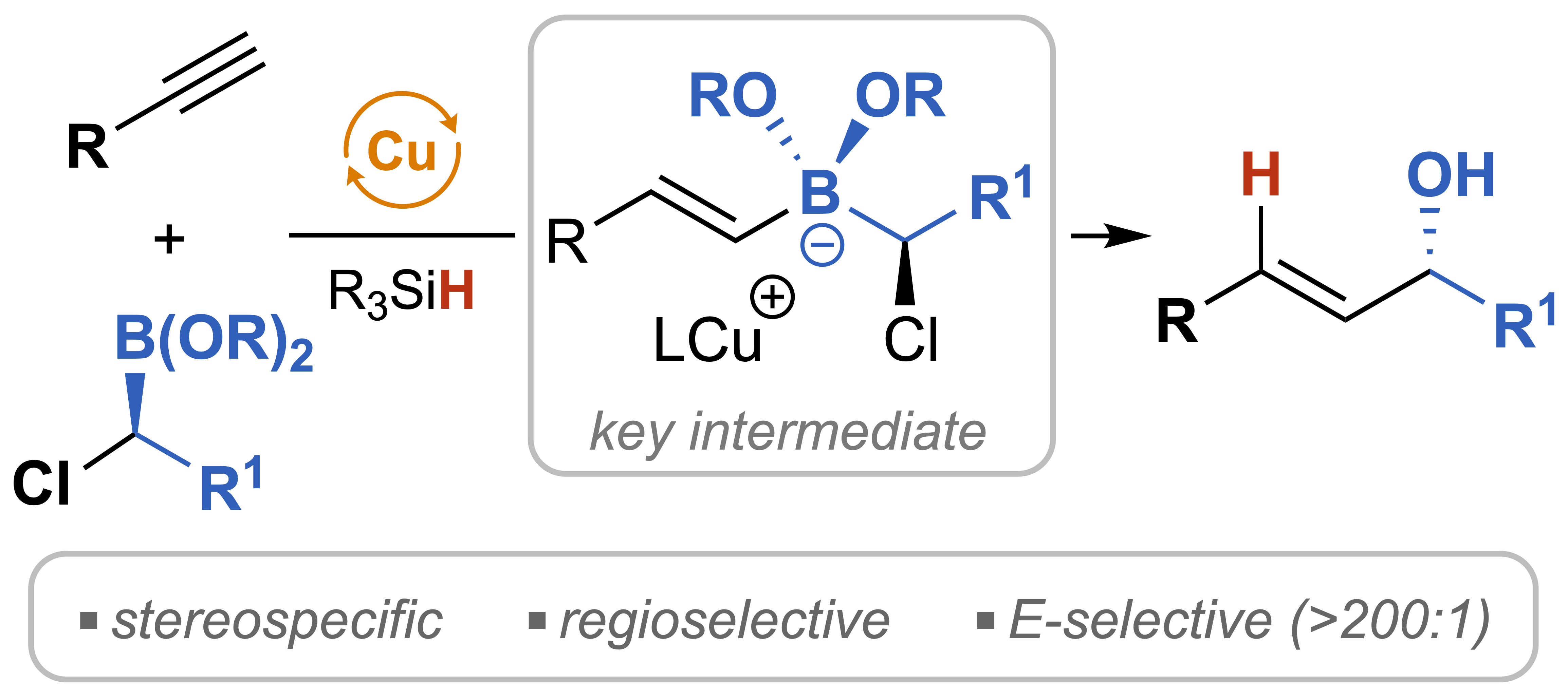
| 44. Shaff, A. B.; Yang, L.; Lee, M. T.; Lalic, G. Stereospecific and Regioselective Synthesis of E-Allylic Alcohols through Reductive Cross Coupling of Terminal Alkynes J. Am. Chem. Soc. 2023, 145, 24615-24624. |link| |
|
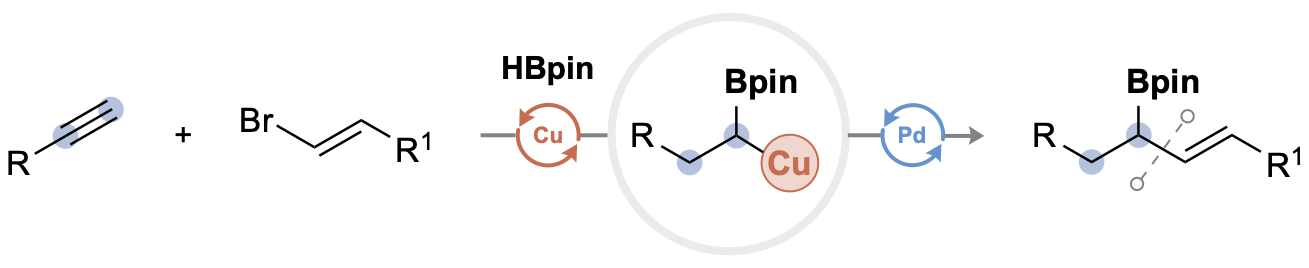
| 43. Baumann, J. E.; Lalic, G. Differential Dihydrofunctionalization: A Dual Catalytic Three-Component Coupling of Alkynes, Alkenyl Bromides, and Pinacolborane Angew. Chem. Int. Ed. 2022, 61, e202206462. |link| |
|
| 42. Lee, M. T.; Lalic, G. Mechanism of Z-Selective Hydroalkylation of Terminal Alkynes J. Am. Chem. Soc. 2021, 143, 16663-16672. |link| |
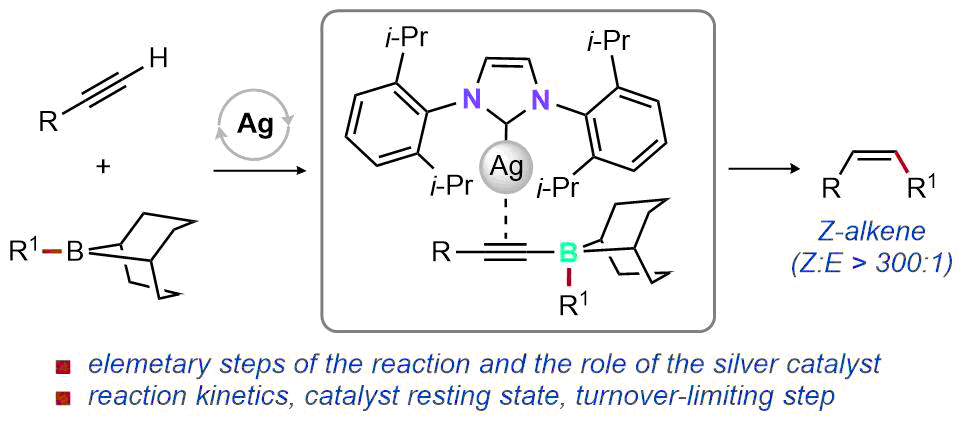
|
| 41. Hazra, A.; Kephart, J. A.; Velian, A.; Lalic, G. Hydroalkylation of Alkynes: Functionalization of the Alkenyl Copper Intermediate through Single Electron Transfer Chemistry J. Am. Chem. Soc. 2021, 143, 7903-7908. |link| |
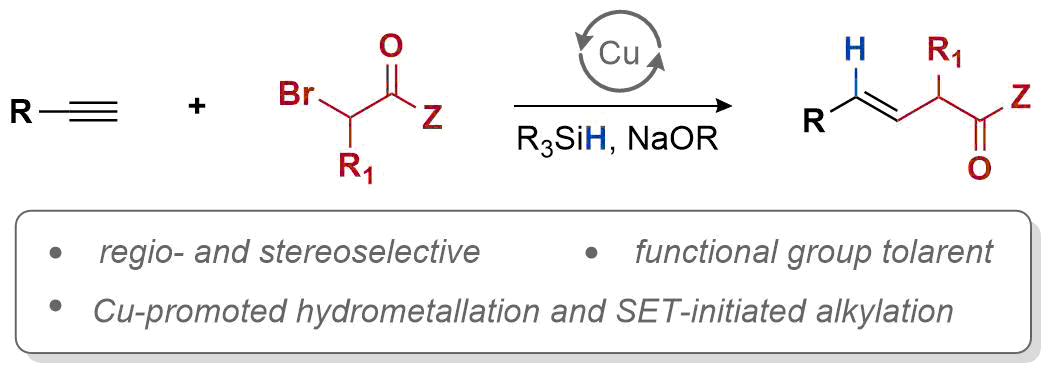
|
| 40. Lee. M. T.; Goodstein, M. B.; Lalic, G. Synthesis of Isomerically Pure (Z)-Alkenes from Terminal Alkynes and Terminal Alkenes: Silver-Catalyzed Hydroalkylation of Alkynes J. Am. Chem. Soc. 2019, 141, 17086-17091. |link| |
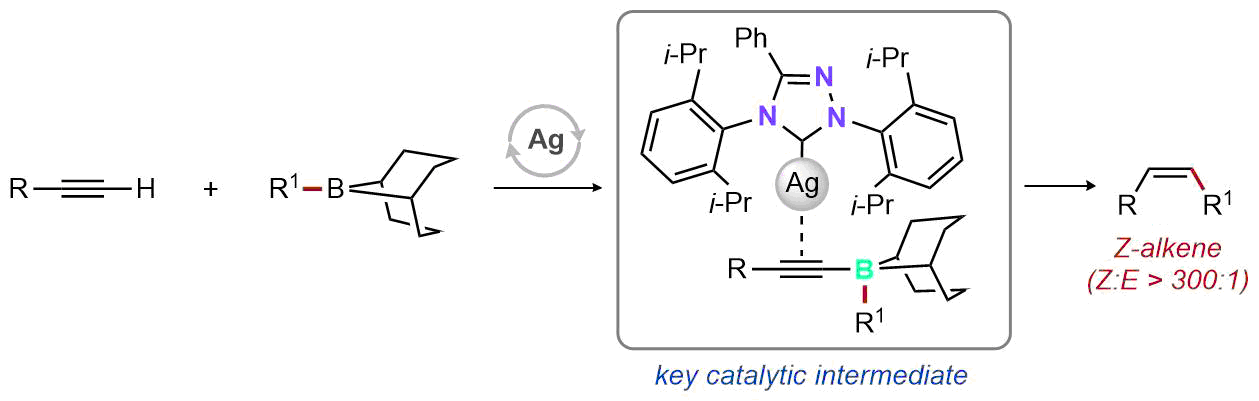
|
| 39. Hazra, A.; Chen, J.; Lalic, G. Stereospecific Synthesis of E-Alkenes through Anti-Markovnikov Hydroalkylation of Terminal Alkynes J. Am. Chem. Soc. 2019, 141, 12464-12469. |link| |
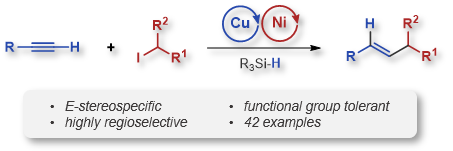
|
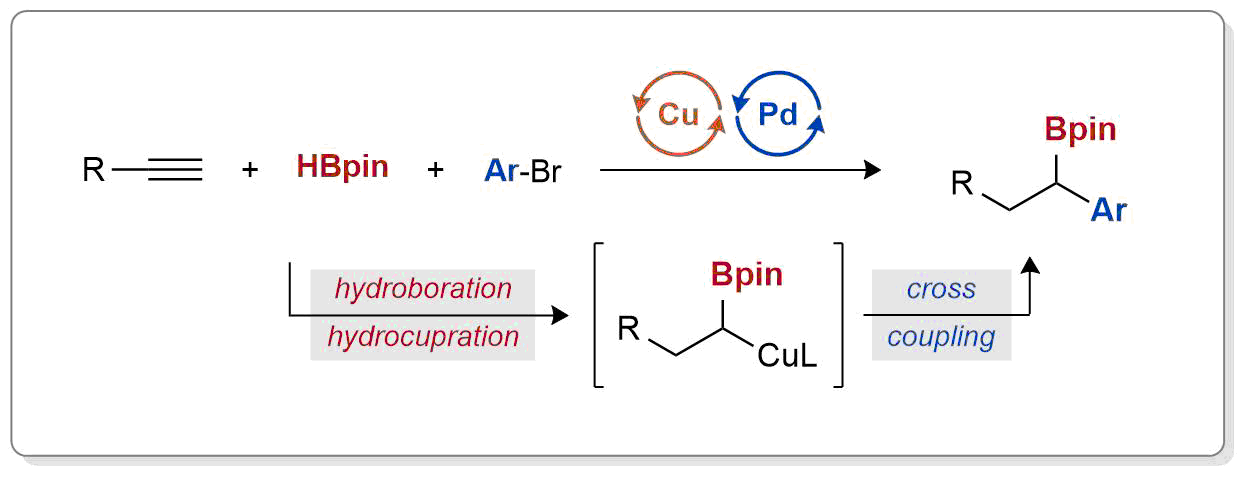
| 38. Armstrong, M. K.; Lalic, G. Differential Dihydrofunctionalization of Terminal Alkynes: Synthesis of Benzylic Alkyl Boronates through Reductive Three-Component Coupling J. Am. Chem. Soc. 2019, 141, 6173-6179. |link| |
|
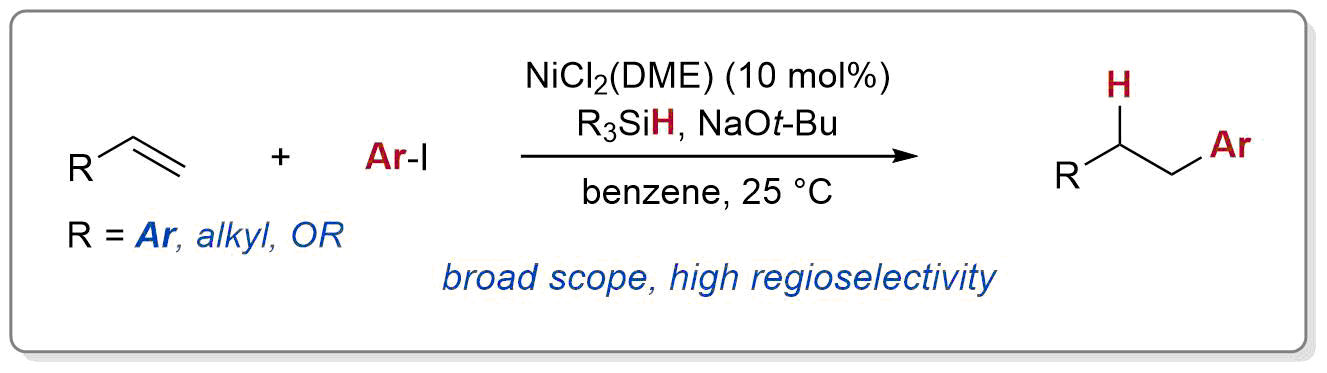
| 37. Nguyen, J.; Chong, A.; Lalic, G. Nickel-Catlayzed Anti-Markovnikov Hydroarylation of Alkenes Chem. Sci. 2019, 10, 3231-3236. (DOI: 10.1039/C8SC05445B) |link| |
|
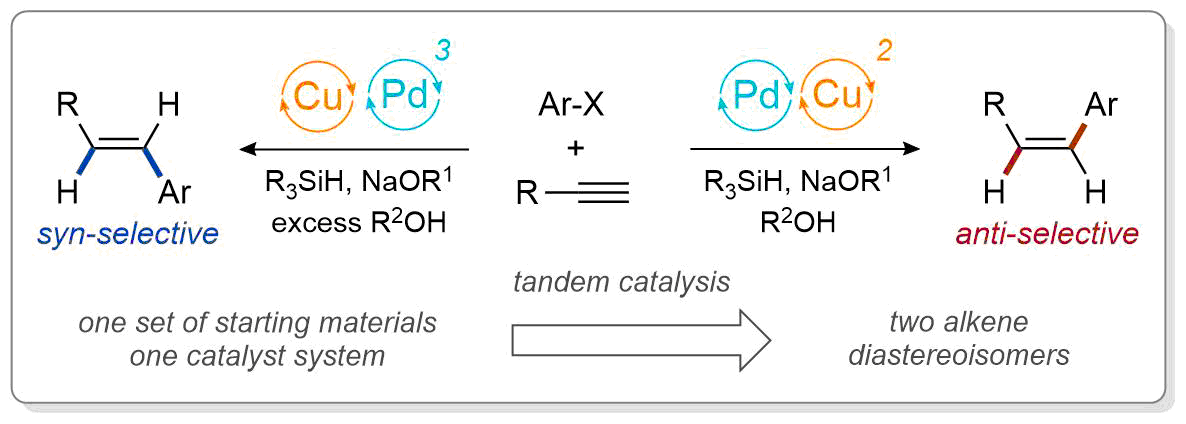
| 36. Armstrong, M. K.; Goodstein, M. B.; Lalic, G. Diastereodivergent Reductive Cross Coupling of Alkynes through Tandem Catalysis: Z- and E-Selective Hydroarylation of Terminal Alkynes J. Am. Chem. Soc. 2018, 140, 10233-10241. (DOI: 10.1021/jacs.8b05113) |link| |
|
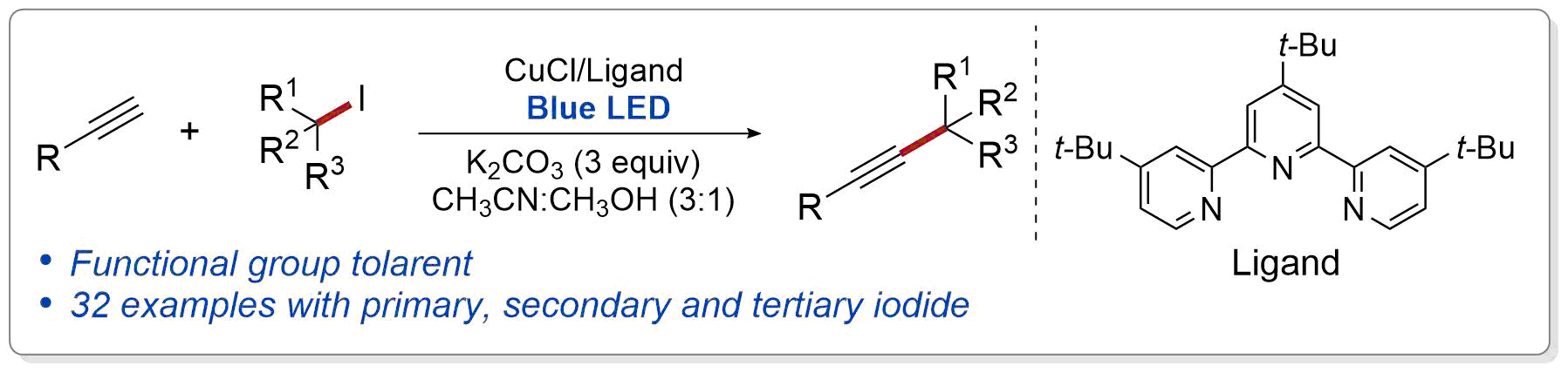
| 35. Hazra, A.; Lee. M. T.; Chiu, J. F.; Lalic, G. Photoinduced Copper-Catalyzed Coupling of Terminal Alkynes and Alkyl Iodides Angew. Chem. Int. Ed. 2018, 57, 5492-5496. (DOI: 10.1002/anie.201709144.) |link| |
|
| 34. Lee, M.; Nguyen, M.; Brandt, C.; Kaminsky, W.; Lalic, G. Catalytic Hydroalkylation of Allenes Angew. Chem. Int. Ed. 2017, 56, 15703-15707 (DOI: 10.1002/anie.201709144.) |link| |
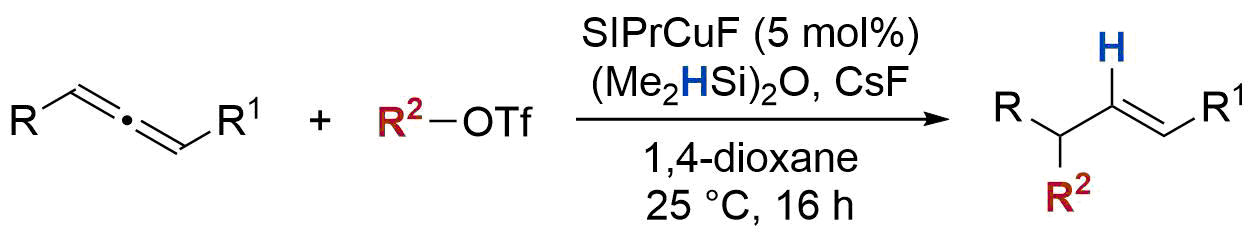
|
| 33. Mailig, M.; Hazra, A.; Armstrong, M. K.; Lalic, G. Catalytic Anti-Markovnikov Hydroallylation of Terminal and Functionalized Internal Alkynes: Synthesis of Skipped Dienes and Trisubstituted Alkenes J. Am. Chem. Soc. 2017, 139, 6969-6977.|link| |
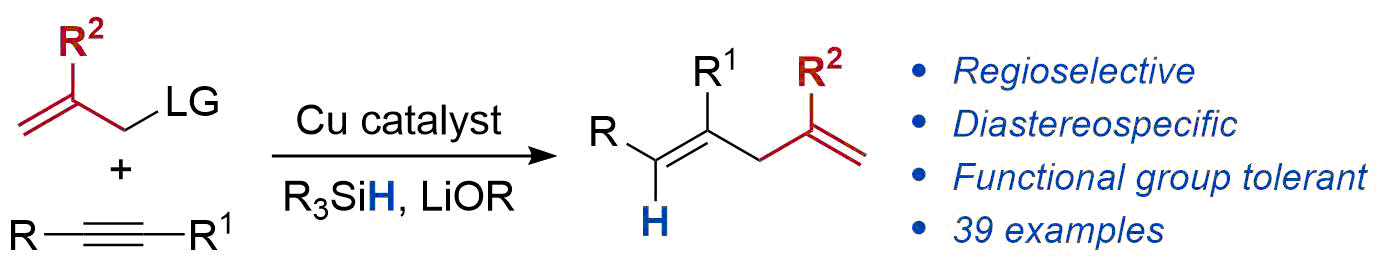
|
| 32. Nguyen, J.; Duncan, N.; Lalic, G. Direct beta-Selective Cross-Coupling of Alkenyl Gold Complexes with Alkyl Electrophiles Eur. J. Org. Chem. 2016, 5803-5806.|link| |

|
| 31. Abraham, J.; Lalic, G.; Sadighi, J. P. Coinage Metal Hydrides: Synthesis, Characterization, and Reactivity Chem. Rev. 2016, 116, 8318-8372.|link| |
|
| 30. Dang, D.; Whittaker, A. M.; Lalic, G. Catalytic Activation of a Single C-F Bond in Trifluoromethyl Arenes Chem. Sci. 2016, 7, 505-509.|link| |
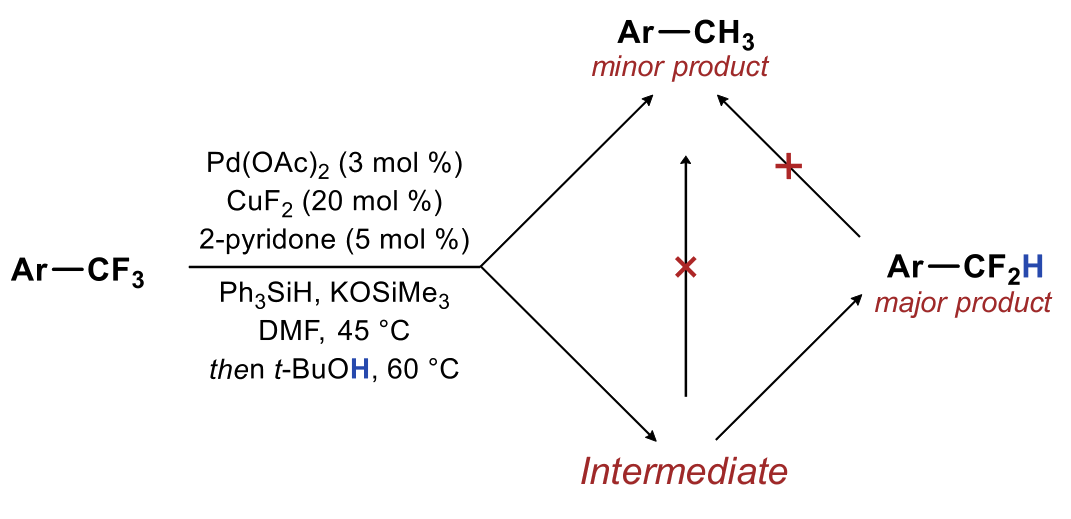
|
| 29. Suess, A. M.; Lalic, G. Copper-Catalyzed Hydrofunctionalization of Alkynes Synlett 2016, 27, 1165-1174.|link| |
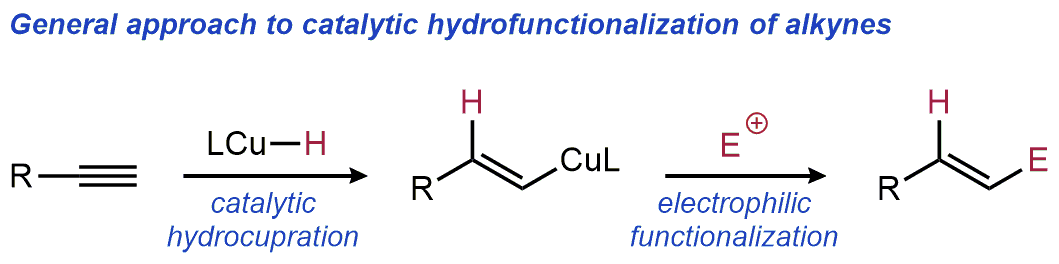
|
| 28. Mailig, M.; Rucker, R. P.; Lalic, G. Practical Catalytic Method for Synthesis of Sterically Hindered Anilines Chem. Commun. 2015, 51, 11048-11051.|link| |
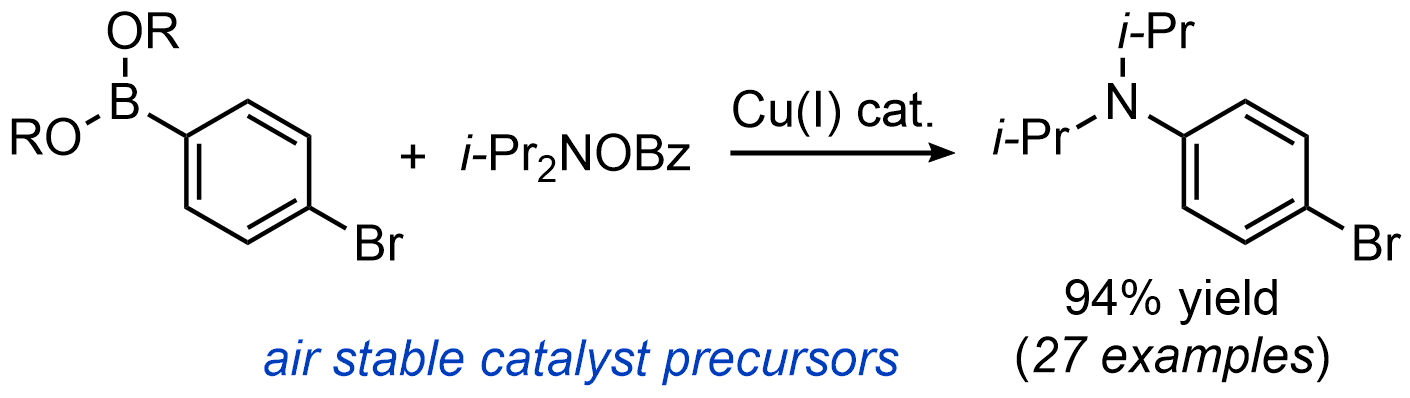
|
| 27. Uehling, M. R.; Suess, A. M.; Lalic, G. Mechanism of Copper-Catalyzed Hydroalkylation of Alkynes: An Unexpected Role of Dinuclear Copper Complexes J. Am. Chem. Soc. 2015, 137, 7747-7753.|link| |
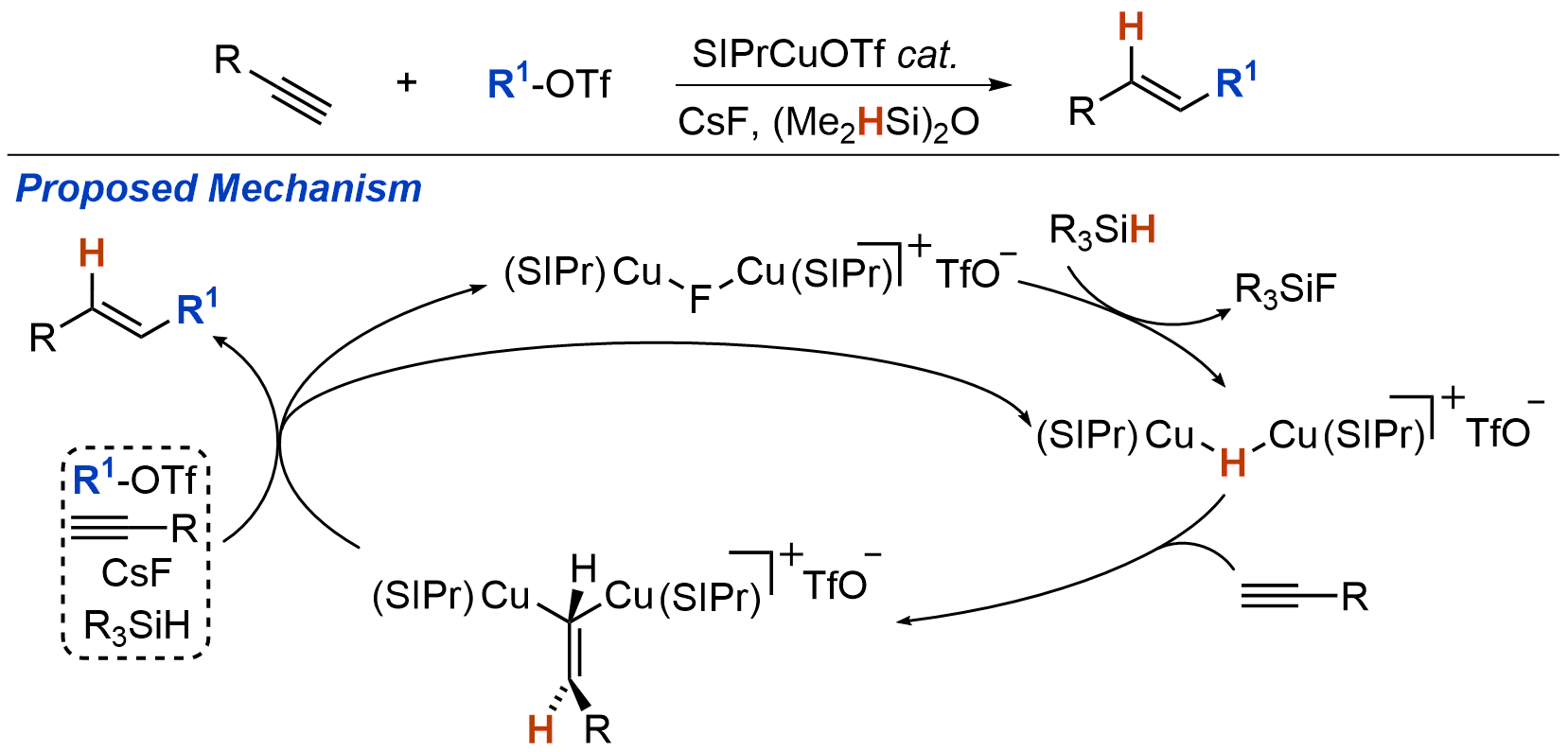
|
| 26. Uehling, M. R.; Suess, A. M.; Lalic, G. Copper-Catalyzed Hydroalkylation of Terminal Alkynes J. Am. Chem. Soc. 2015, 137, 1424-1427.|link| |
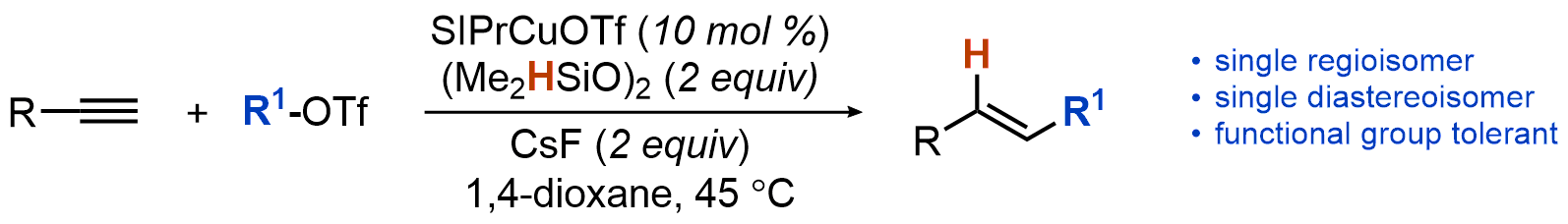
|
| 25. Uehling, M. R.; Rucker, R. P.; Lalic, G. Catalytic Anti-Markovnikov Hydrobromination of Alkynes J. Am. Chem. Soc. 2014, 136, 8799-8803.|link| |
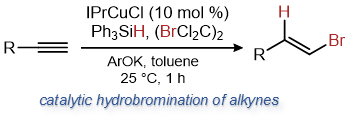
|
| 24. Dang, H.; Mailig, M.; Lalic, G. Mild Copper-Catalyzed Fluorination of Alkyl Triflates with Potassium Fluoride Angew. Chem. Int. Ed. 2014, 53, 6473-6476.|link| |
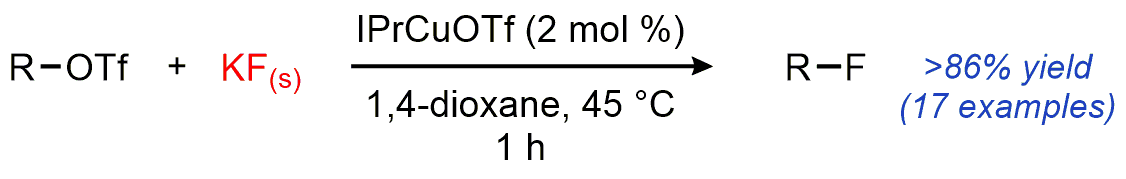
|
| 23. Cox, N.; Dang, H.; Whittaker, A. M.; Lalic, G. NHC-copper hydrides as chemoselective reducing agents: catalytic reduction of alkynes, alkyl triflates, and alkyl halides Tetrahedron 2014, 27-28, 4219-4231.|link| |
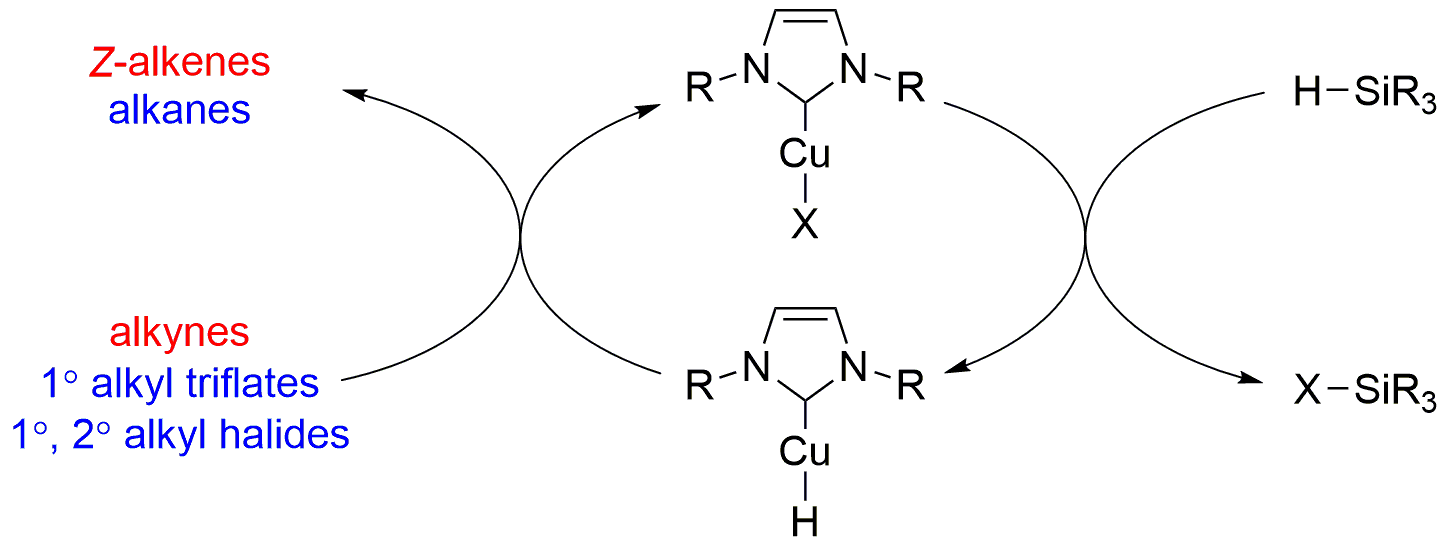
|
| 22. Dang, H.; Cox, N.; Lalic, G. Copper-Catalyzed Reduction of Alkyl Triflates and Iodides: An Efficient Method for the Deoxygenation of Primary and Secondary Alcohols Angew. Chem. Int. Ed. 2014, 53, 752.|link| |
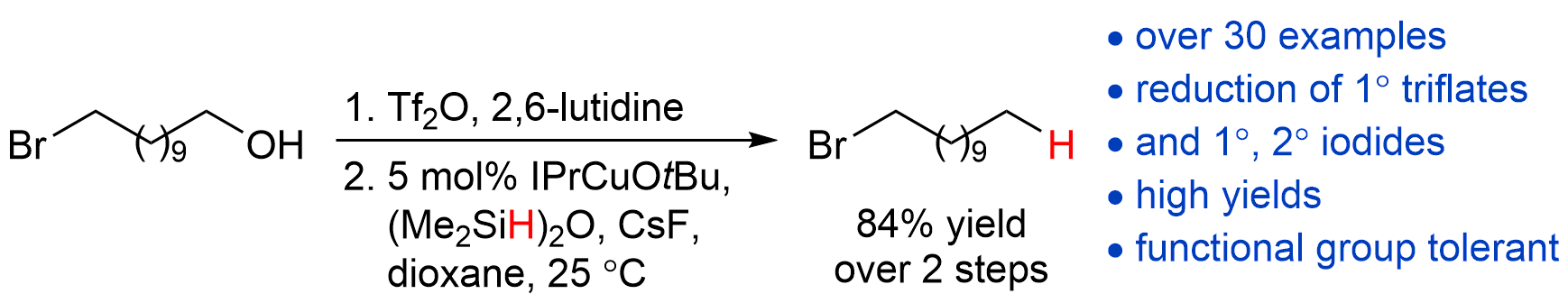
|
| 21. Cox, N.; Uehling, M. R.; Haelsig, K. T.; Lalic, G. Catalytic Asymmetric Synthesis of Cyclic Ethers Containing an a-Tetrasubstituted Stereocenter Angew. Chem. Int. Ed. 2013, 52, 4878. (Highlighted in SYNFACTS).|link| |
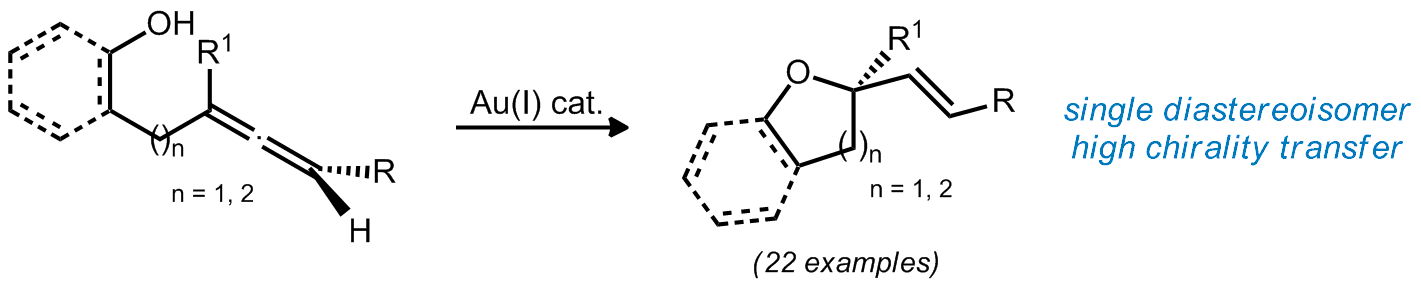
|
| 20. Whittaker, A. M.; Lalic, G. Monophasic Catalytic System for the Selective Semireduction of Alkynes Org. Lett. 2013, 15, 1113.(Highlighted in SYNFACTS)|link| |
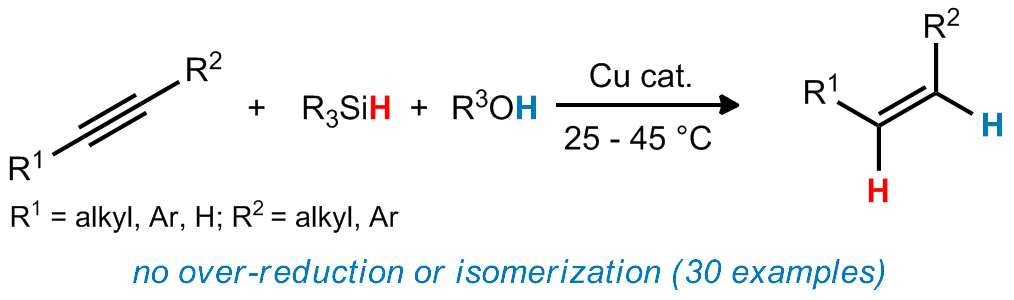
|
| 19. Rucker, R. P.; Lalic, G. Copper-Catalyzed Electrophilic Amination of Organoboron Compounds Synlett 2013, 24, 269. (Invited synpacts article)|link| |
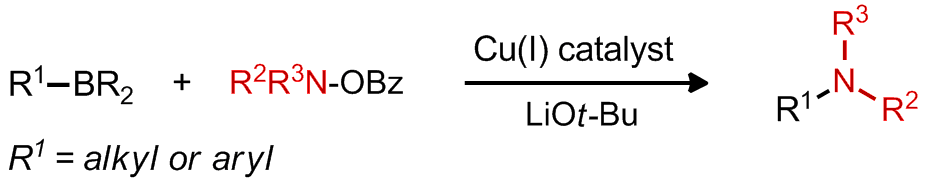
|
| 18. Rucker, R. P.; Whittaker, A. M.; Dang, H.; Lalic, G. Synthesis of Tertiary Alkyl Amines from Terminal Alkenes: Copper-Catalyzed Amination of Alkyl Boranes J. Am. Chem. Soc. 2012, 134, 6571. |link| |
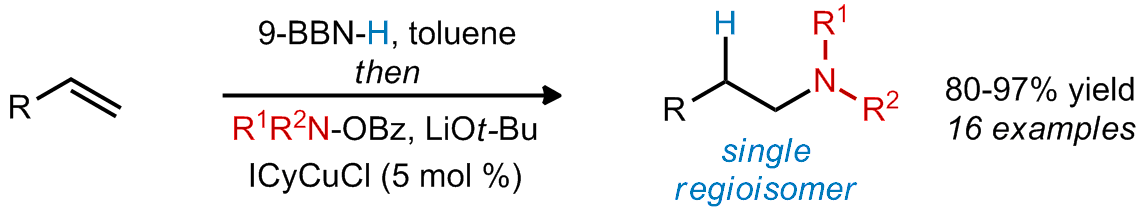
|
| 17. Rucker, R. P.; Whittaker, A. M.; Dang, H.; Lalic, G. Synthesis of Hindered Anilines: Copper-Catalyzed Electrophilic Amination of Aryl Boronic Esters Angew. Chem. Int. Ed. 2012, 51, 3953. |link| |
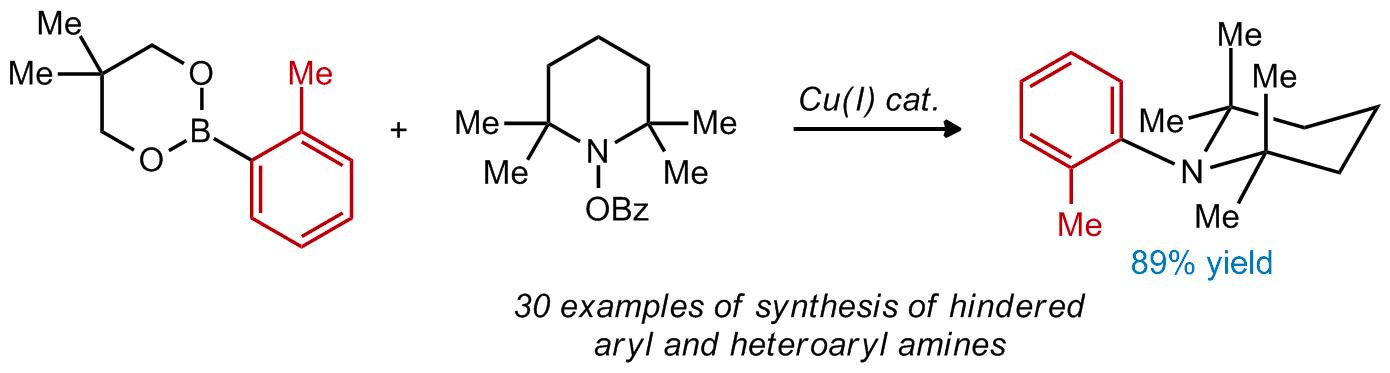
|
| 16. Uehling, M. R.; Marionni, S. T.; and Lalic, G. Asymmetric Synthesis of Trisubstituted Allenes: Copper-Catalyzed Alkylation and Arylation of Propargylic Phosphates Org. Lett. 2012, 14, 362. |link| |
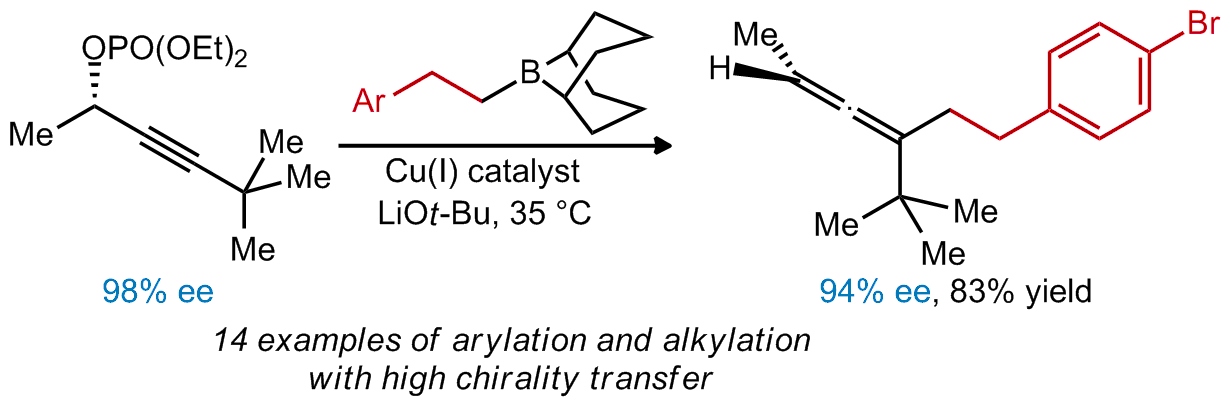
|
| 15. Whittaker, A. M.; Rucker, R. P.; Lalic, G. Catalytic SN2'-Selective Substitution of Allylic Chlorides With Arylboronic Esters. Org. Lett. 2010, 12, 3216. |link| |
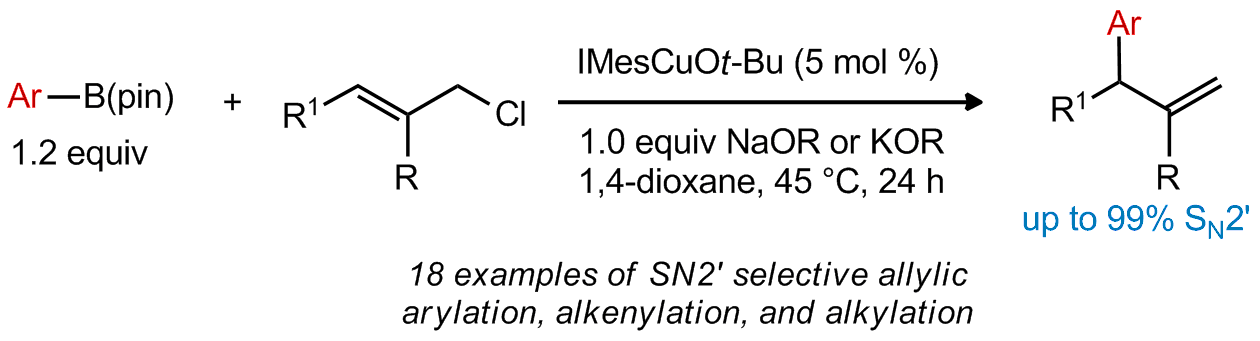
|
| Before UW |
| 14. Lalic G.; Corey E. J. Enantioselective Rhodium(I)-Triethylamine Catalyzed Addition of Potassium Isopropenyltrifluoroborate to Enones. Tetrahedron Lett. 2008, 12, 3216. |
| 13. Lalic G.; Krinsky J. L.; Bergman R. G. The Scope and the Mechanism of SN2' Substitution Reactions of a Monomeric Imidozirconium Complex With Allylic Electrophiles. J. Am. Chem. Soc. 2008, 130, 4459. |
| 12. Lalic G.; Corey E. J. An Effective Enantioselective Route to the Platensimycin Core. Org. Lett. 2007, 9, 4921. |
| 11. Fox R. J.; Lalic G.; Bergman R. G. Regio- and Stereospecific Formation of Protected Allylic Alcohols via Zirconium-Mediated SN2' Substitution of Allylic Chlorides. J. Am. Chem. Soc. 2007, 129, 14144. |
| 10. Lalic G.; Blum S. A.; Bergman R. G. Zirconium-Mediated SN2' Substitution of Allylic Ethers: Regio- and Stereospecific Formation of Protected Allylic Amines. J. Am. Chem. Soc. 2005, 127, 16790. |
| 9. Magdziak D.; Lalic G.; Myung Lee H.; Fortner K. C.; Aloise, A. D.; Shair M. D. Catalytic Enantioselective Thioester Aldol Reactions That are Compatible With Protic Functional Groups. J. Am. Chem. Soc. 2005, 127, 7284. |
|
8. Xu K.; Lalic G.; Sheehan S. M.; Shair M. D.
Dynamic Kinetic Resolution during a Cascade Reaction on Substrates with Chiral All-Carbon Quaternary Centers. Angew. Chem. Int. Ed. 2005, 44, 2259. |
| 7. Lalic G.; Aloise A. D.; Shair M. D. An Exceptionally Mild Thioester Aldol Reaction Inspired by Polyketide Biosynthesis. J. Am. Chem. Soc. 2003, 125, 2852. |
|
6. Burke M. D.; Lalic G.
Teaching Target-Oriented and Diversity-Oriented Organic Synthesis at Harvard University. Chem. Biol. 2002, 9, 535. |
|
5. Korbel G. A.; Lalic G.; Shair M. D.
Reaction Microarrays: A Method for Rapidly Determining the Enantiomeric Excess of Thousands of Samples. J. Am. Chem. Soc. 2001, 123, 361. |
|
4. Lalic G.; Petrovski Z.; Galonic D.; Matovic R.; Saicic R. N.
Alkylation of Carbonyl Compounds in the TiCl4-promoted Reaction of Trimethylsilyl Enol Ethers with Epoxides. Tetrahedron 2001, 57, 583. |
|
3. Sheehan S. M.; Lalic G.; Chen J. S.; Shair M. D.
A Highly Efficient and Convergent Reaction for the Synthesis of Bridgehead Enone-Containing Polycyclic Ring Systems. Angew. Chem. Int. Ed. 2000, 39, 2714. |
|
2. Lalic G.; Petrovski Z.; Galonic D.; Matovic R.; Saicic R. N.
Alkylation of Carbonyl Compounds in the TiCl4-promoted Reaction of Trimethylsilyl Enol Ethers with Ethylene Oxide. Tetrahedron Lett. 2000, 41, 763. |
|
1. Barton D. H. R.; Lalic G.; Smith J. A.
The Selective Functionalization of Saturated Hydrocarbons. Part 42. Further Studies in Selective Phenylselenation. Tetrahedron 1998, 54, 1725. |
