The following is a collection of problems in organic and organometallic chemistry
collected by Boydston, Lalic, and Michael groups.
The keywords for each problem can be made visible by selecting the space immediatly following the "Keywords:" heading.
The collection can be searched for a particular keyword using CTRL+F function of your web browser.
For a list of keywords currently in the collection click here
For the chemdraw template click here
Last update 08.30.2022
| Problem 95. Provide a mechanism for the following transformations. Contributor: J.E. Baumann Source: Angew. Chem. Int. Ed. 2022, e202210592. Click here for a worksheet covering the total synthesis of (-)-kopsinine |
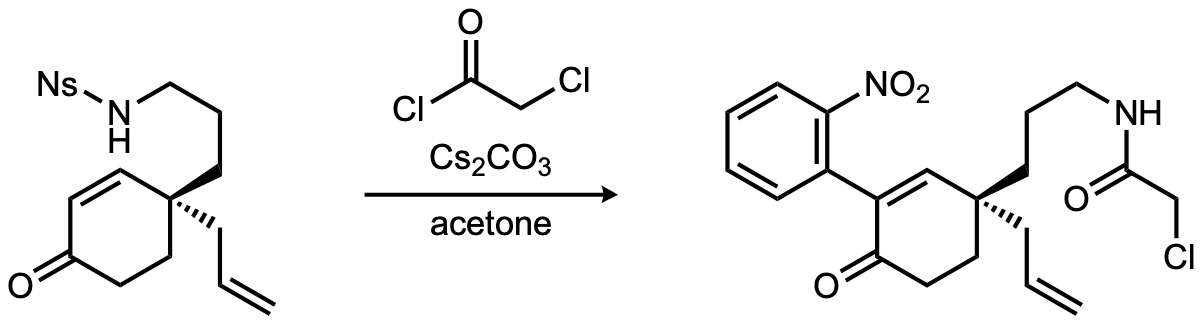
|
| Problem 94. Provide a mechanism for the following transformations. Contributor: AJ Boydston Source: Nature 2012, 490, 208. Keywords: Diels-Alder, cycloaddition |
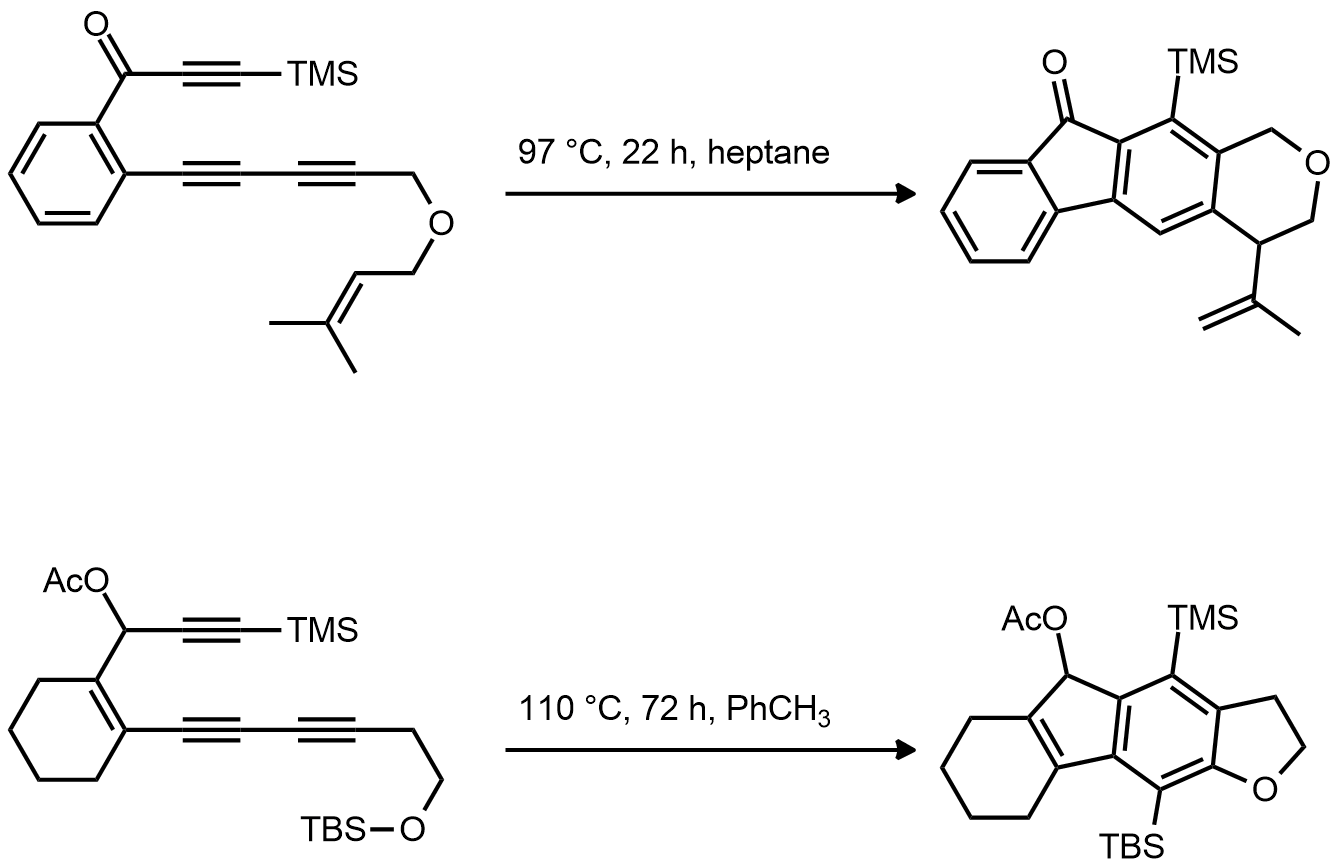
|
| Problem 93. Provide a mechanism for the following transformation. Contributor: AJ Boydston Source: Org. Lett. DOI: 10.1021/ol400317v. Keywords: photoredox catalysis |
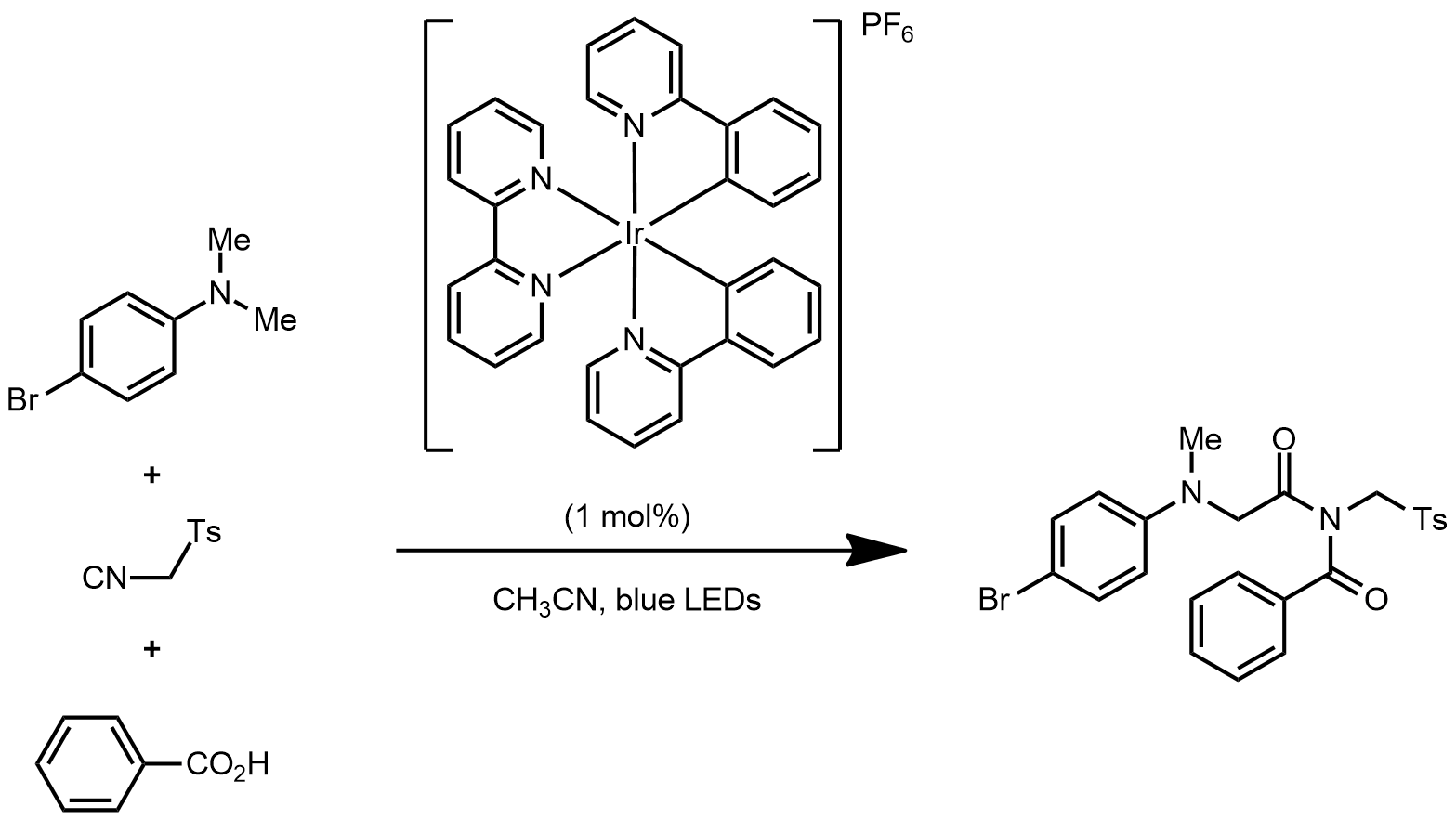
|
| Problem 92. Provide a mechanism for the following transformations. Contributor: AJ Boydston Source: Org. Lett. 2013, 15, 914. Keywords: Shapiro reaction, rearrangement |
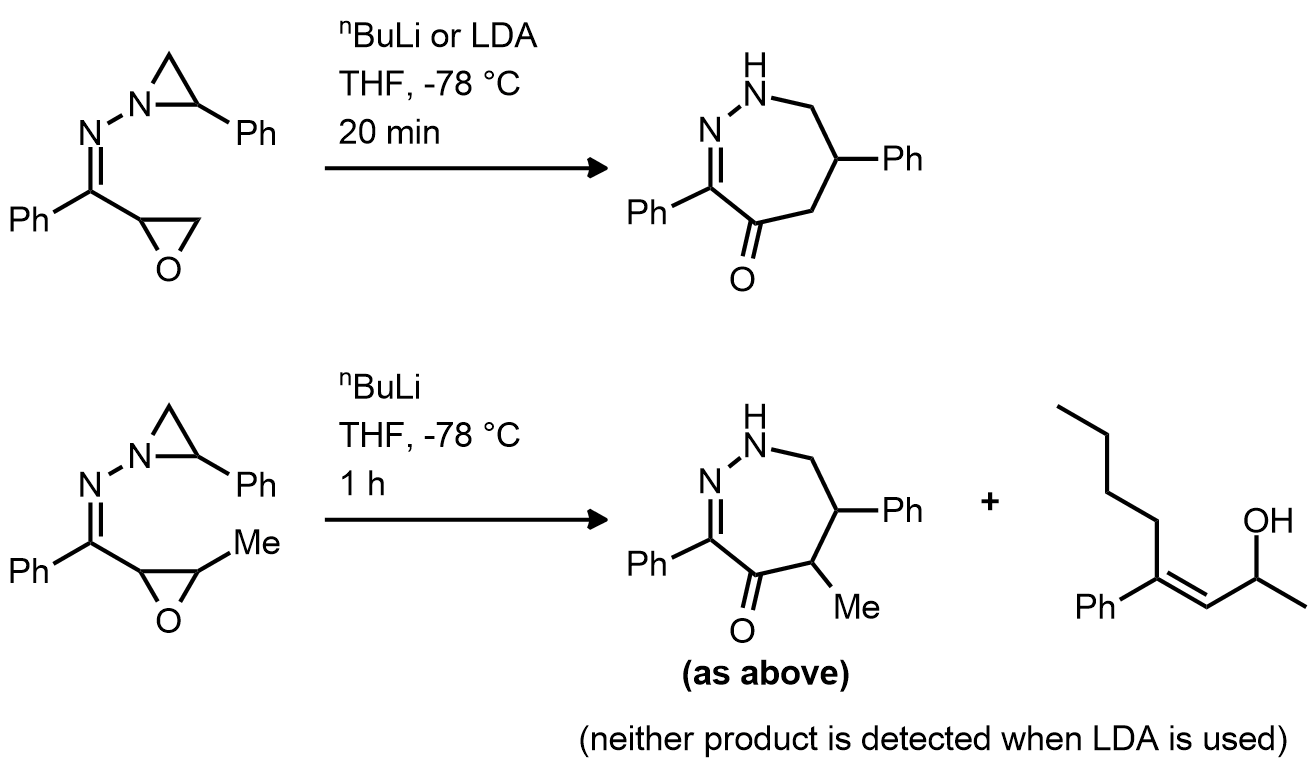
|
| Problem 91. Provide a mechanism for the following transformations. Contributor: AJ Boydston Source: Org. Lett. 2013, 15, 476. Keywords: Piancatelli rearrangement |
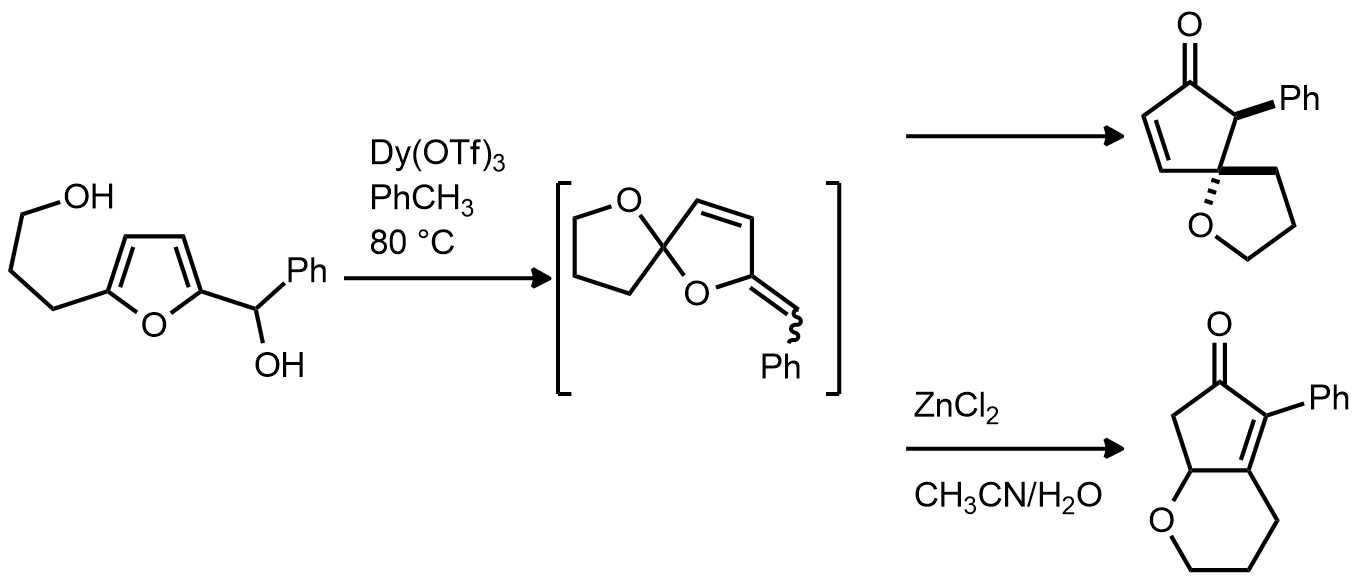
|
| Problem 90. Provide a mechanism for the following transformation. Contributor: AJ Boydston Source: Angew. Chem. Int. Ed. 2013, 52, 4016. Keywords: cyclopropanation |
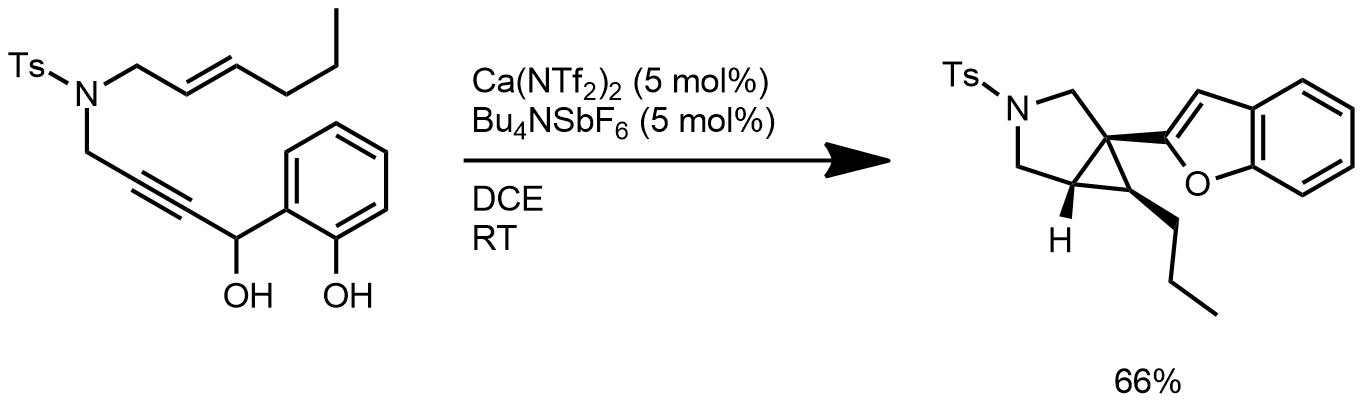
|
| Problem 89. Provide products and mechanisms for the following reactions. Contributor: M Larsen Source: Eur. J. Org. Chem. (DOI: 10.1002/ejoc.201201516). Keywords: ene-yne metathesis |
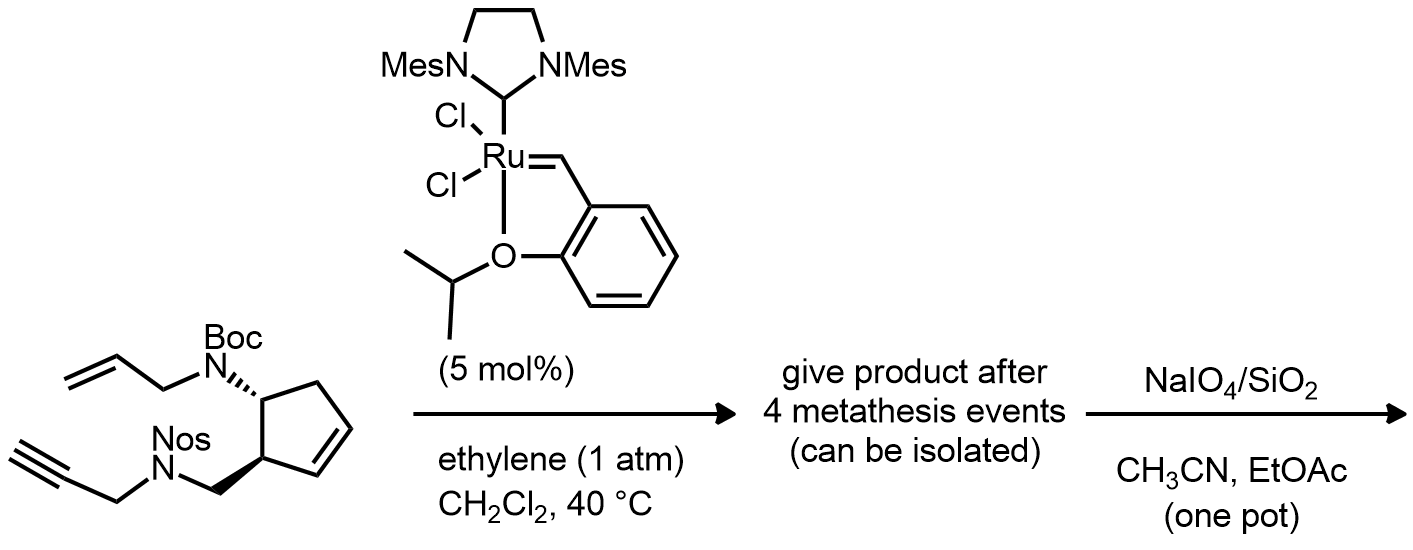
|
| Problem 88. Provide a mechanism for the following transformation. Contributor: AJ Boydston Source: Chem. Commun. 2013, 49, 1193. Keywords: sigmatropic rearrangement |
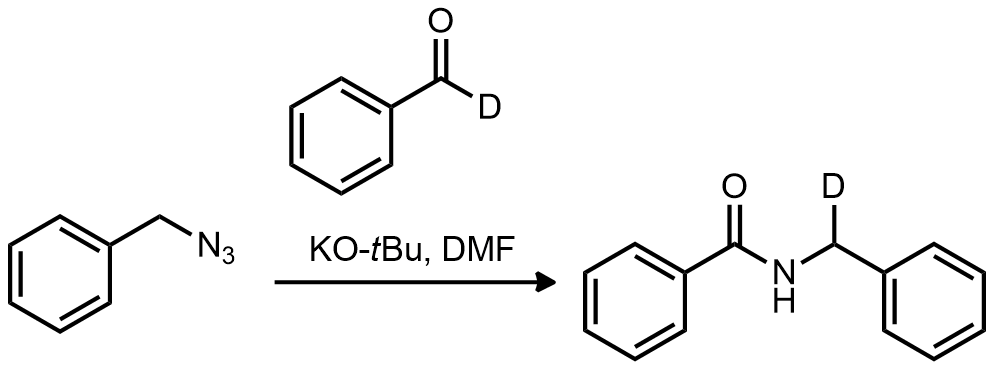
|
| Problem 87. Provide the product and a plausible mechanism for the following transformation Contributor: AJ Boydston Source: Org. Lett. 2013, 15, 602. Keywords: Lossen rearrangement |
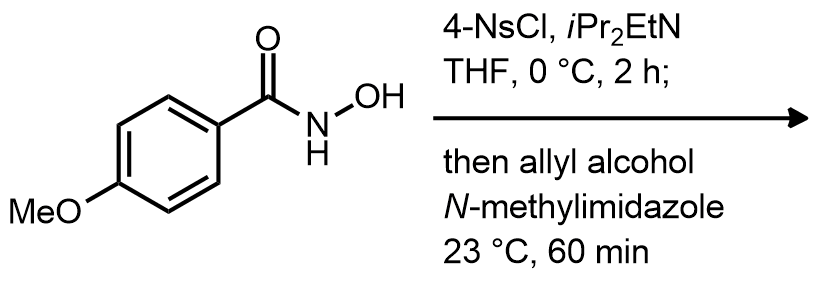
|
| Problem 86. Provide an explanation for the observed product ratios. Contributor: AJ Boydston Source: Org. Lett. 2012, 14, 256. Keywords: organocatalysis, cyclization |
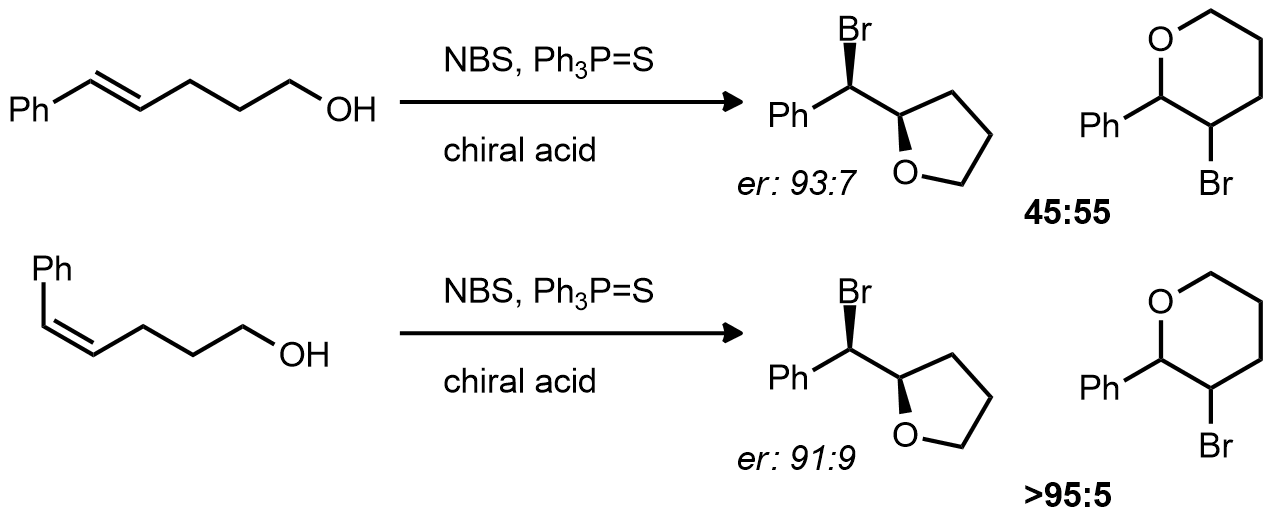
|
| Problem 85. Propose an explanation for the catalytic activity. Contributor: AJ Boydston Source: J. Am. Chem. Soc. 2012, 134, 12693. Keywords: photochemical, organocatalysis, photoswitch |
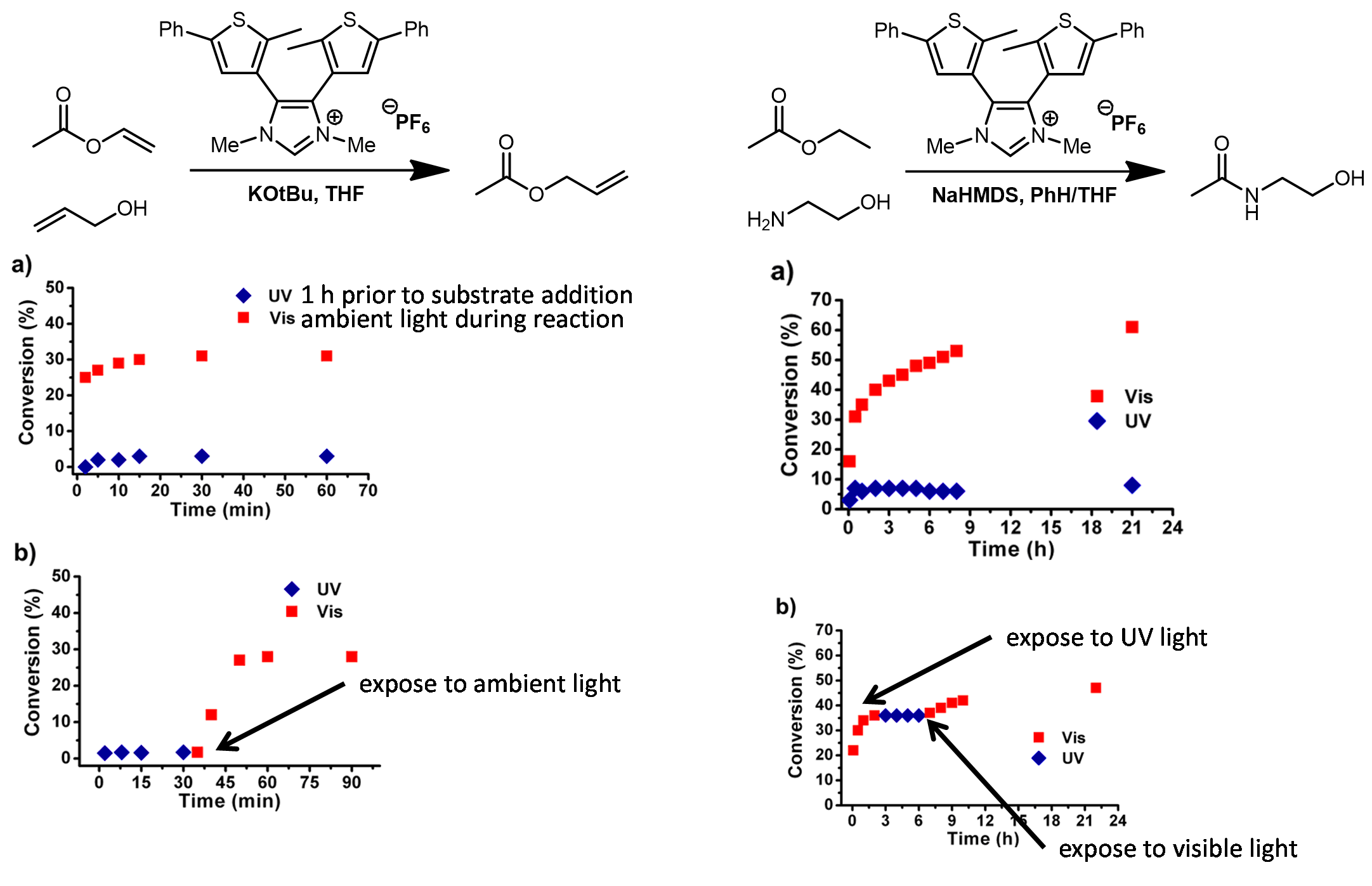
|
| Problem 84. Provide a mechanism for the following transformation. Contributor: AJ Boydston Source: Angew. Chem. Int. Ed. 2011, 50, 4445. Keywords: Witkop reaction, photochemical, cytclization, electron transfer |
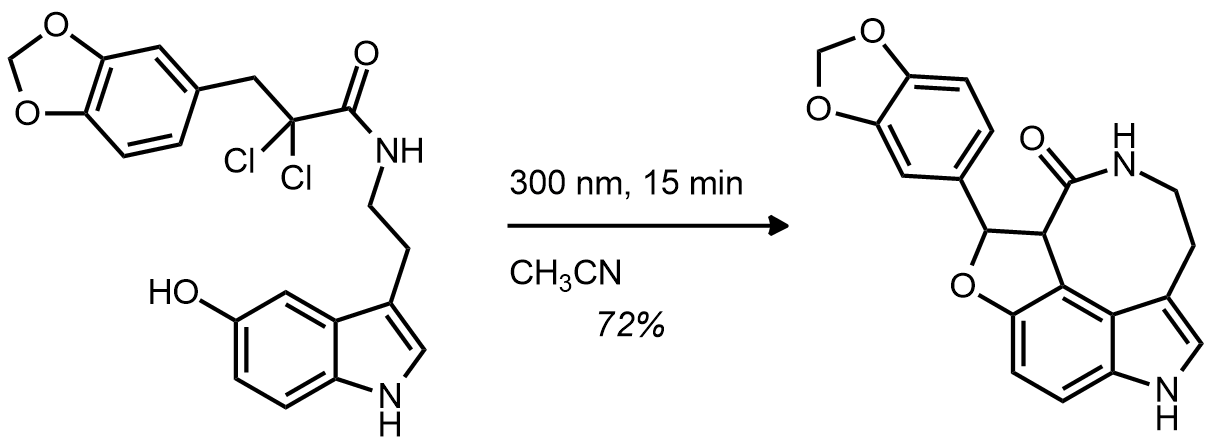
|
| Problem 83. Provide a mechanism for the following transformation. Contributor: AJ Boydston Source: J. Org. Chem. 2012, 77, 6327. Keywords: cyclization |
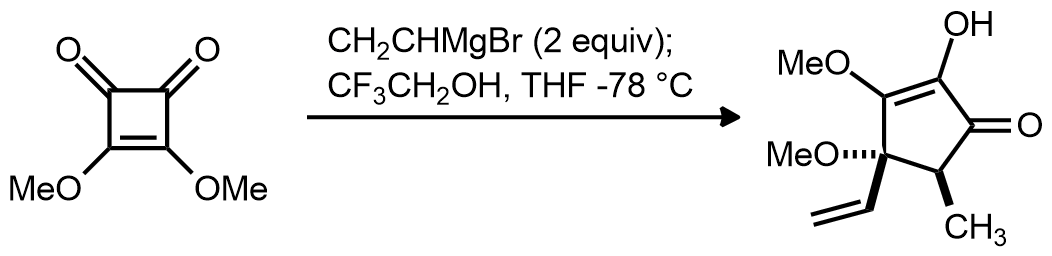
|
| Problem 82. Provide a mechanism for the following transformation. Contributor: G Lalic Source: Tetrahedron Lett. 1968, 5609. Keywords: radical |
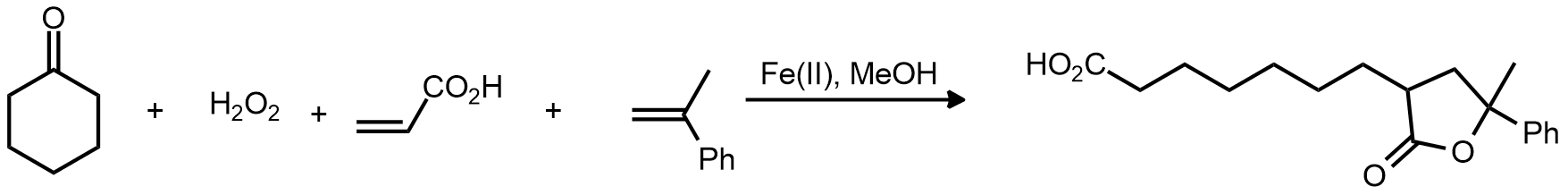
|
| Problem 81. Provide a mechanism for the following transformation. Contributor: G Lalic Source: Tetrahedron Lett. 1992, 2157. Keywords: radical |
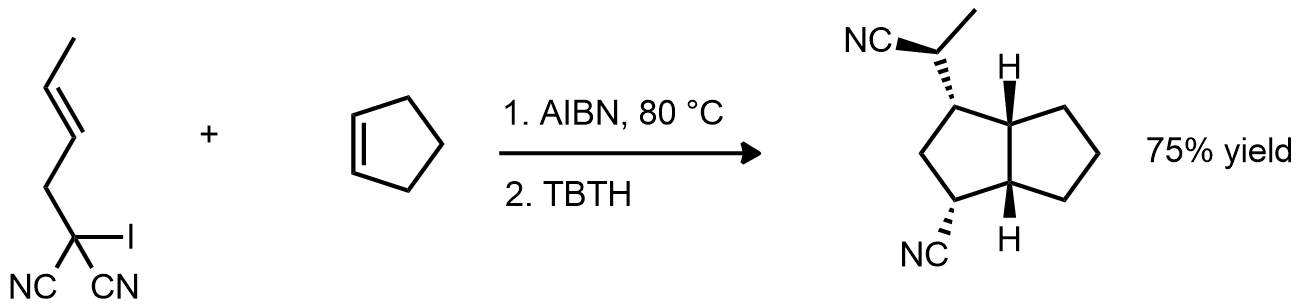
|
| Problem 80. Provide a mechanism for the following transformation. Contributor: G Lalic Source: Tetrahedron Lett. 1993, 127. Keywords: radical |
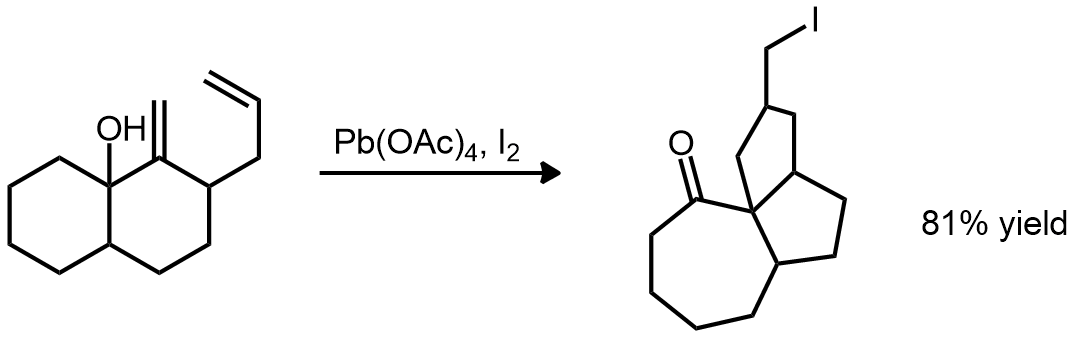
|
| Problem 79. Provide a mechanism for the following transformation. Contributor: G Lalic Source: J. Org. Chem. 1990, 55, 5181. Keywords: radical |
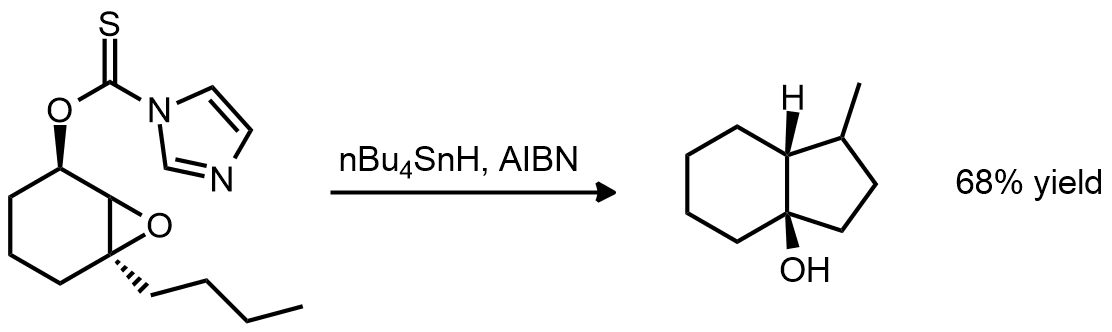
|
| Problem 78. Provide a mechanism for the following transformation. Contributor: G Lalic Source: J. Am. Chem. Soc. 1990, 112, 5601. Keywords: radical |
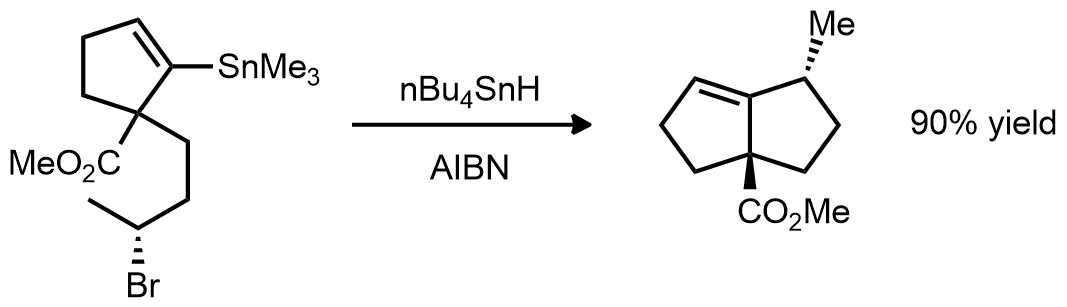
|
| Problem 77. Provide a mechanism for the following transformation. Contributor: G Lalic Source: Chem. Commun. 1988, 81. Keywords: radical |
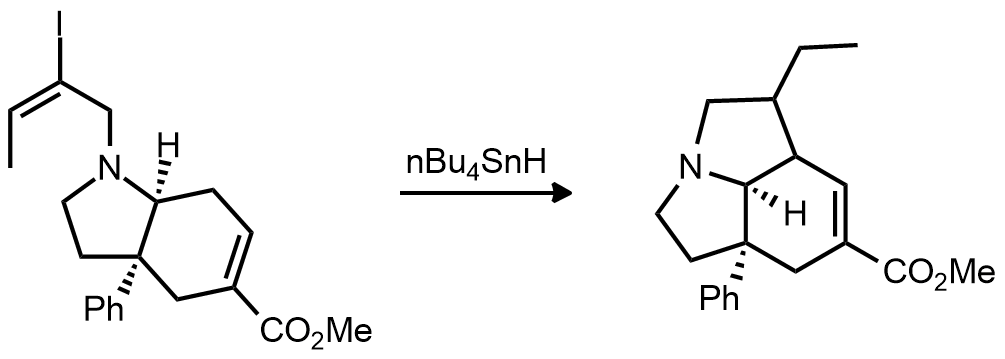
|
| Problem 76. Provide a mechanism for the following transformation. Contributor: G Lalic Source: Tetrahedron Lett. 1983, 15. Keywords: radical |
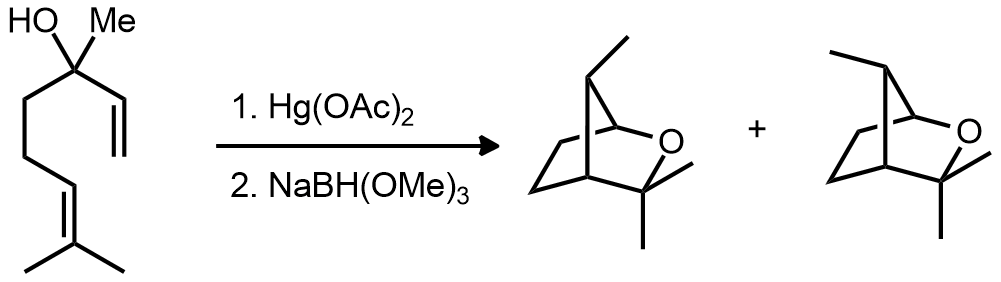
|
| Problem 75. Provide a mechanism for the following transformations. Contributor: G Lalic Source: J. Org. Chem. 1974, 39, 3456. Keywords: radical |
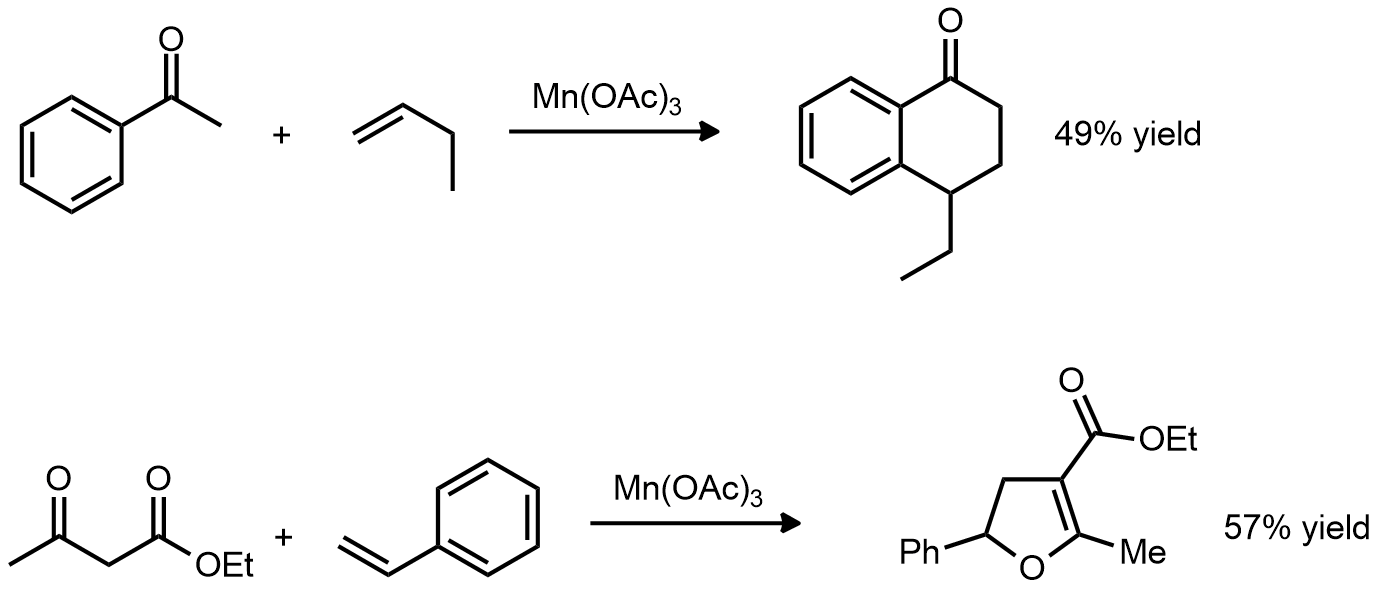
|
| Problem 74. Provide a mechanism for the formation of all the products. How would you supress the formation of the byproducts? Contributor: G Lalic Source: Keywords: radical |
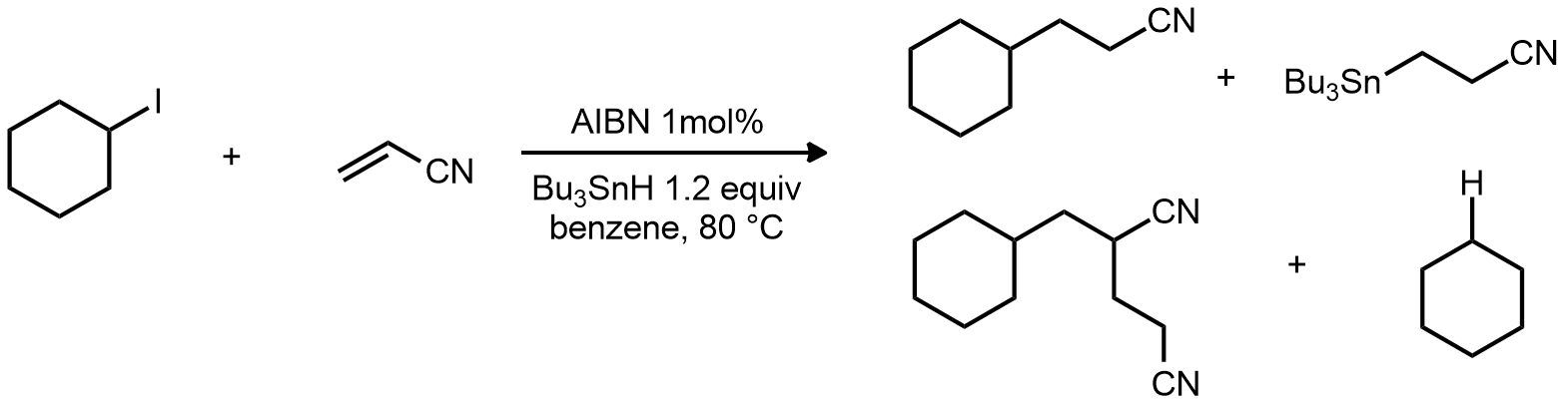
|
| Problem 73. Provide a mechanism for the following transformations. Contributor: G Lalic Source: J. Am. Chem. Soc. 2013, 135, 66. Keywords: CH activation |
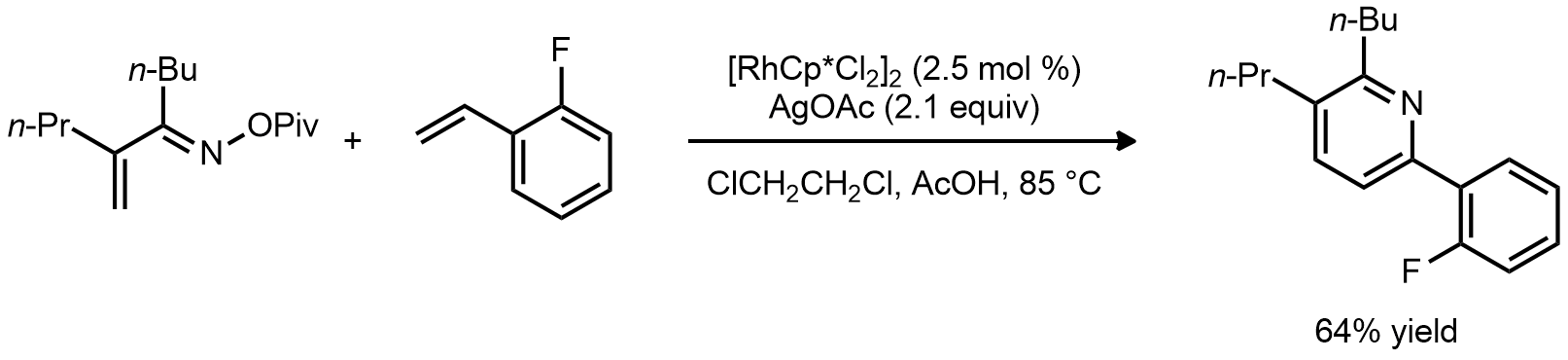
|
| Problem 72. Provide a mechanism for the following transformations. Contributor: G Lalic Source: J. Am. Chem. Soc. 2007, 129, 10096. Keywords: iminium |
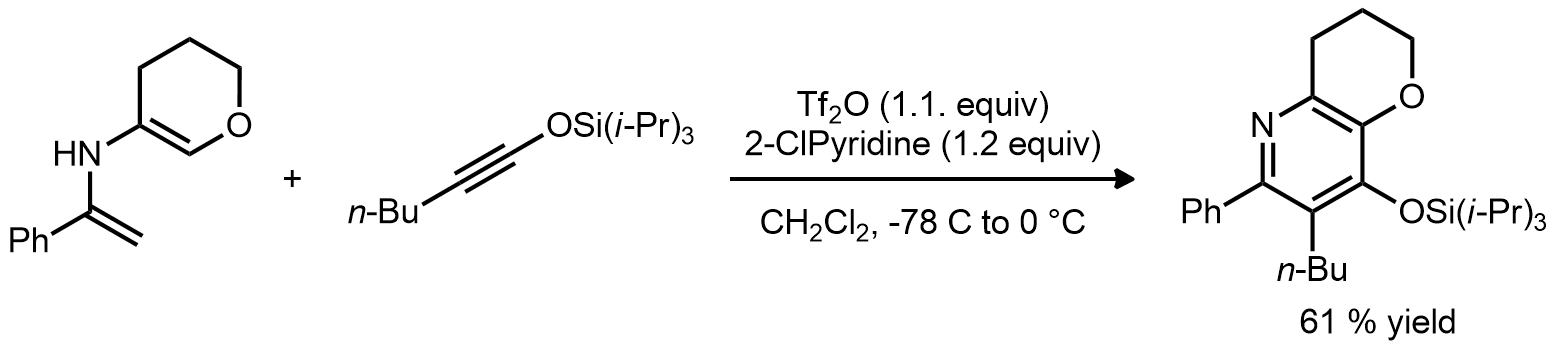
|
| Problem 71. Provide a mechanism for the following transformations. Contributor: G Lalic Source: J. Am. Chem. Soc. 2011, 133, 6411. Keywords: radical addition |
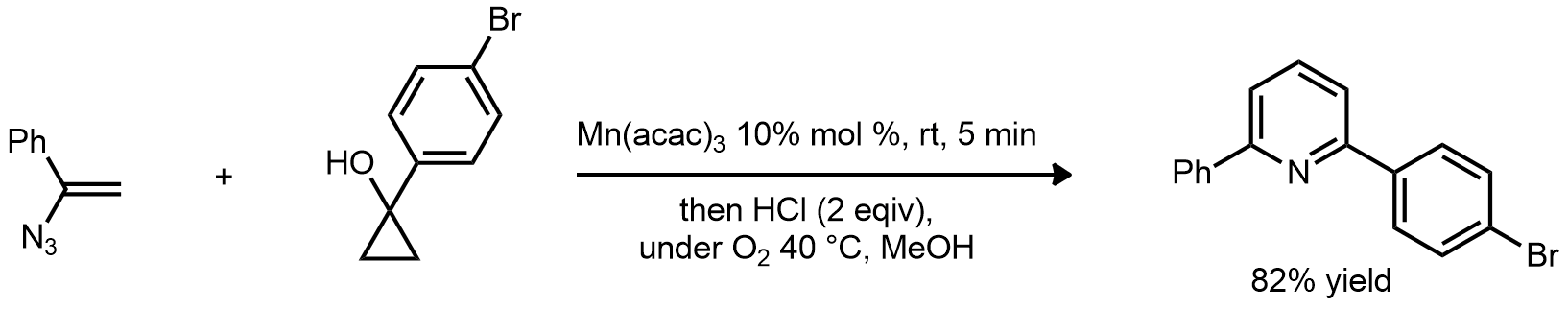
|
| Problem 70. Provide a mechanism for the following transformations. Contributor: G Lalic Source: J. Am. Chem. Soc. 2011, 133, 18018. Keywords: cycloisomerization |
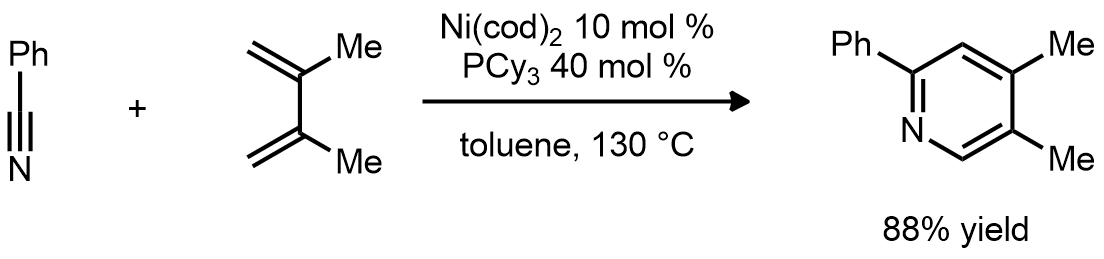
|
| Problem 69. Provide a mechanism for the following transformations. Contributor: G Lalic Source: J. Am. Chem. Soc. 2008, 130, 6918. Keywords: electrocyclic reaction |
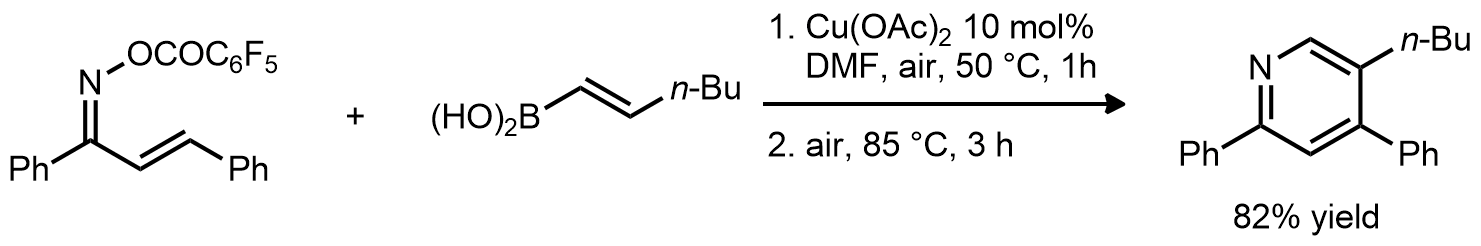
|
| Problem 68. Provide a mechanism for the following transformations. Contributor: G Lalic Source: J. Am. Chem. Soc. 2012, 134, 9078. Keywords: carbanion |
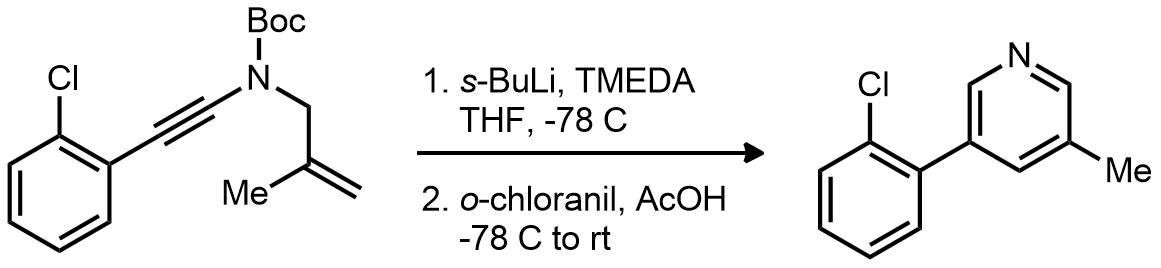
|
| Problem 67. Provide a mechanism for the following transformations. Contributor: G Lalic Source: J. Am. Chem. Soc. 2008, 130, 8602. Keywords: carbene, sigmatropic rearrangement |
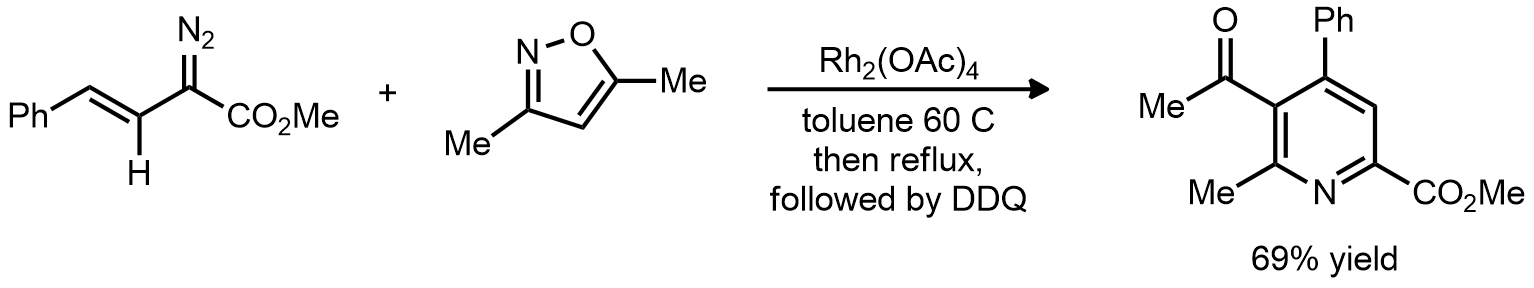
|
| Problem 66. Provide a mechanism for the following transformations. Contributor: AJ Boydston Source: J. Am. Chem. Soc. 2011, 133, 14785. Keywords: Tsuji-Trost allylation, Claisen condensation |
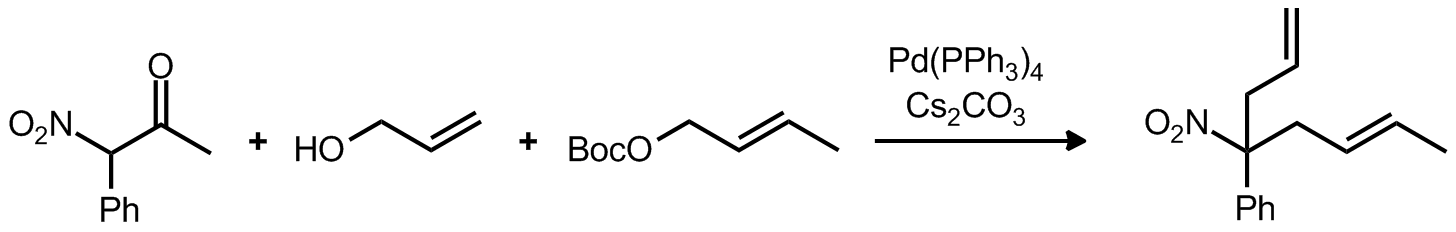
|
| Problem 65. Propose a synthesis of the target molecule from each starting material. Contributor: AJ Boydston Source: J. Org. Chem. 2012, 77, 9422. Keywords: synthesis |
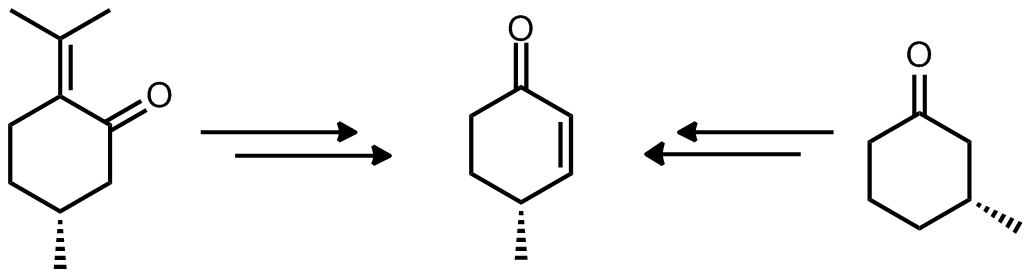
|
| Problem 64. Provide a mechanism for the following transformations. Contributor: AJ Boydston Source: Org. Lett. 2012, 14, 264. Keywords: Eschenmoser-Tanabe fragmentation |
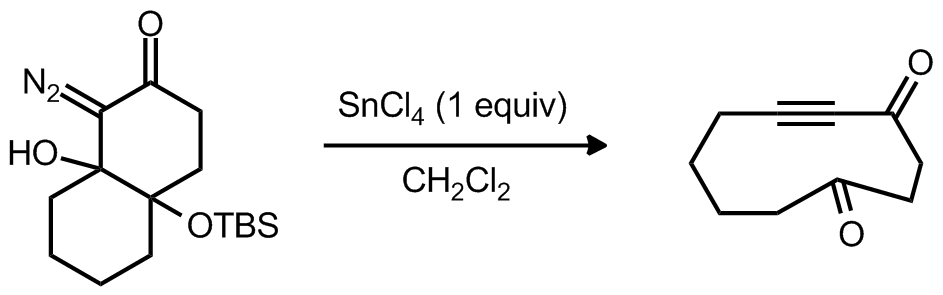
|
| Problem 63. Provide a mechanism for the following transformations. Contributor: AJ Boydston Source: Tetrahedron 1993, 49, 10501. Keywords: aldol,Grob fragmentation |
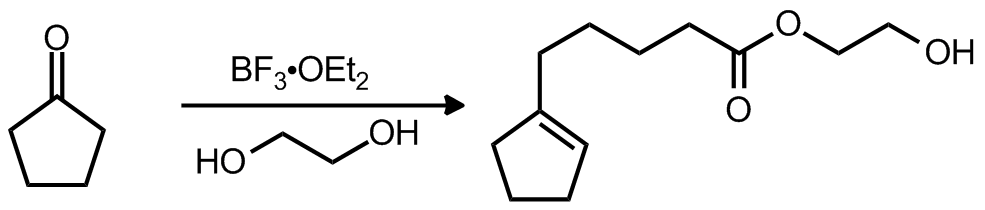
|
| Problem 62. Provide a mechanism for the following transformations. Contributor: G. Lalic Source: Org. Lett. 2012, 14, 126. Keywords: retro 2+2, 4+2, cheletropic reaction, 3+2 |
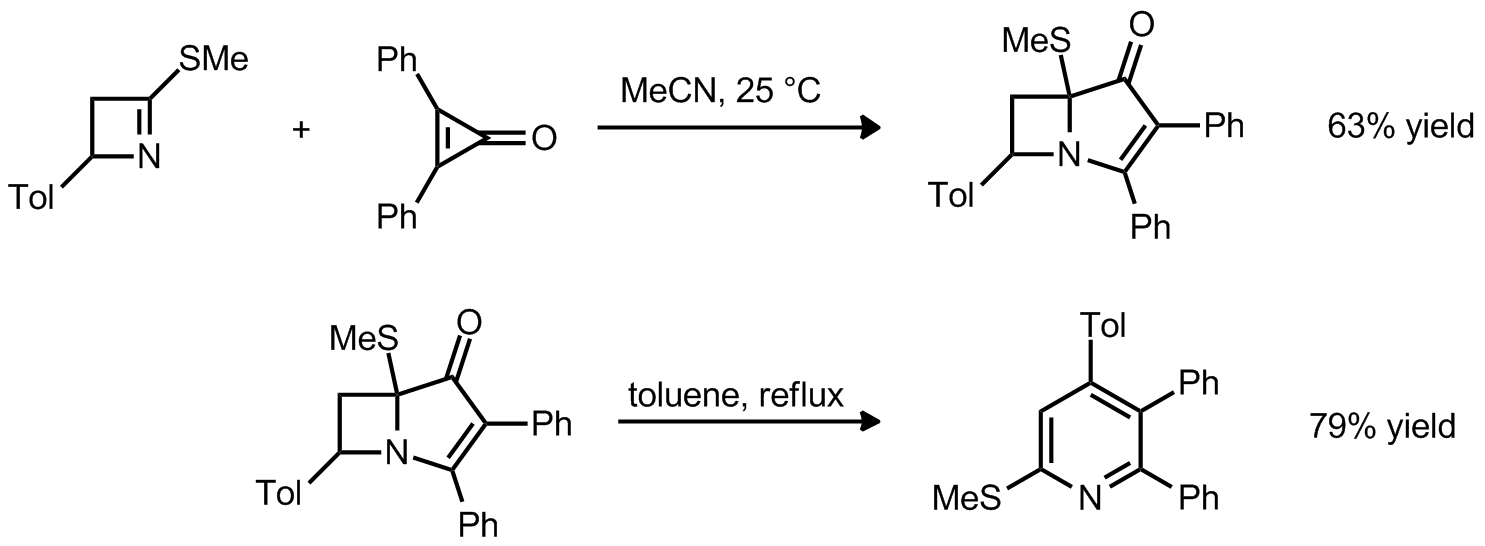
|
| Problem 61. Provide a mechanism for the following transformations. Provide a structure of A. Contributor: G. Lalic Source: J. Am. Chem. Soc. 2012, 134, 7286. Keywords: rearrangement |
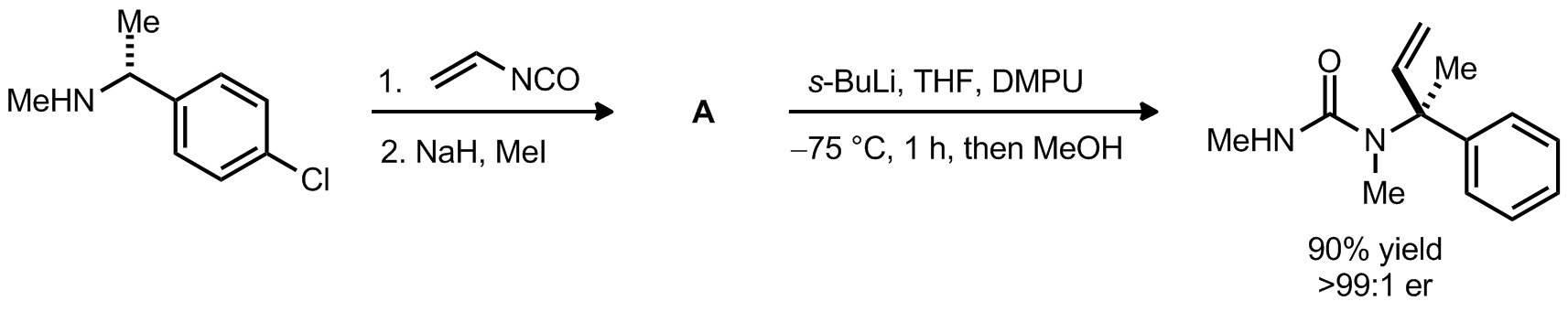
|
| Problem 60. Provide an explanation for the following observation. Contributor: G. Lalic Source: J. Am. Chem. Soc. 2012, 134, 7262. Keywords: nitrene, CH activation |
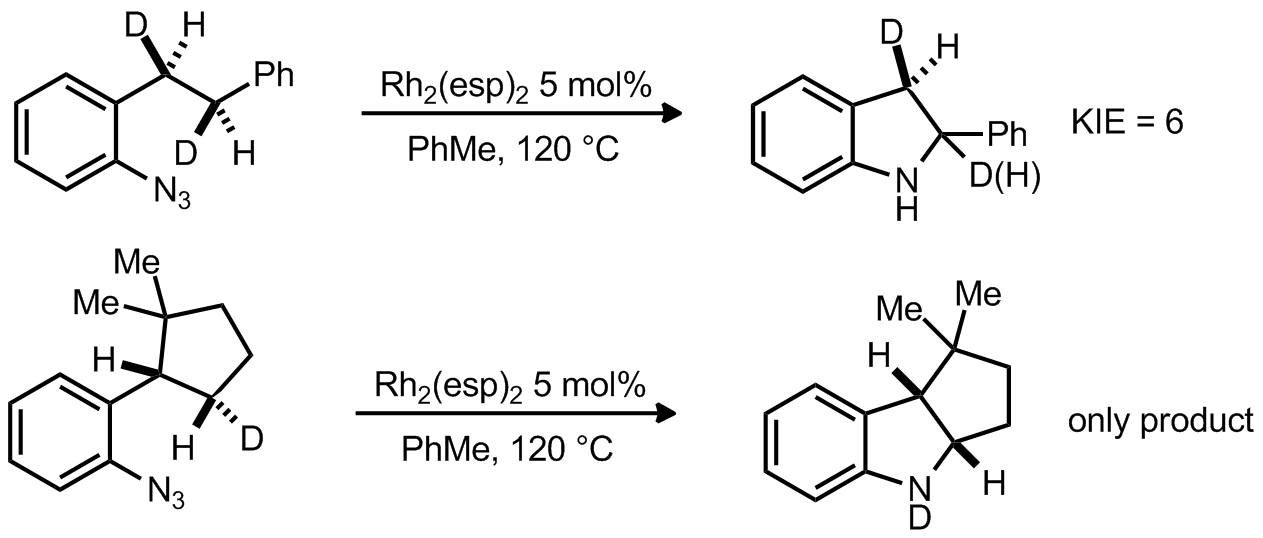
|
| Problem 59. Provide a mechanism for the following transformation. Contributor: G. Lalic Source: J. Am. Chem. Soc. 2012, 134, 7262. Keywords: nitrene, CH activation |
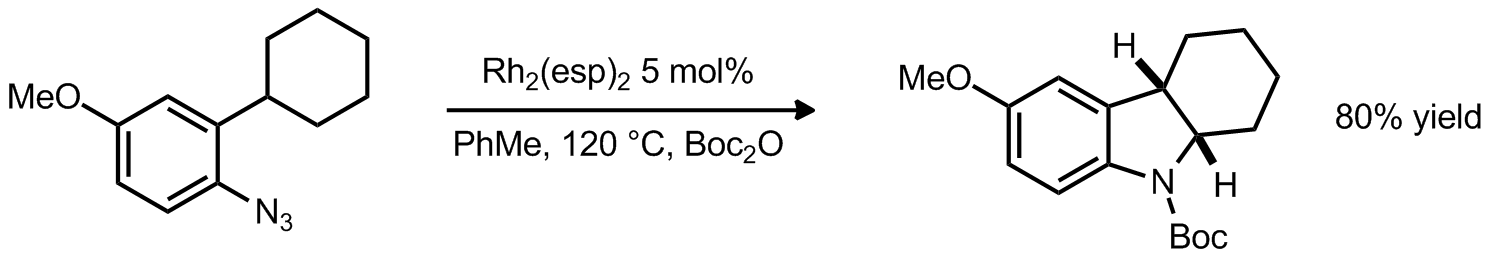
|
| Problem 58. Provide a mechanism for the following transformation. Provide an explanation for the observed diastereoselectivity. Contributor: G. Lalic Source: J. Am. Chem. Soc. 2012, 134, 6928. Keywords: 1,2 sigmatropic rearrangement, electrophilic activation |
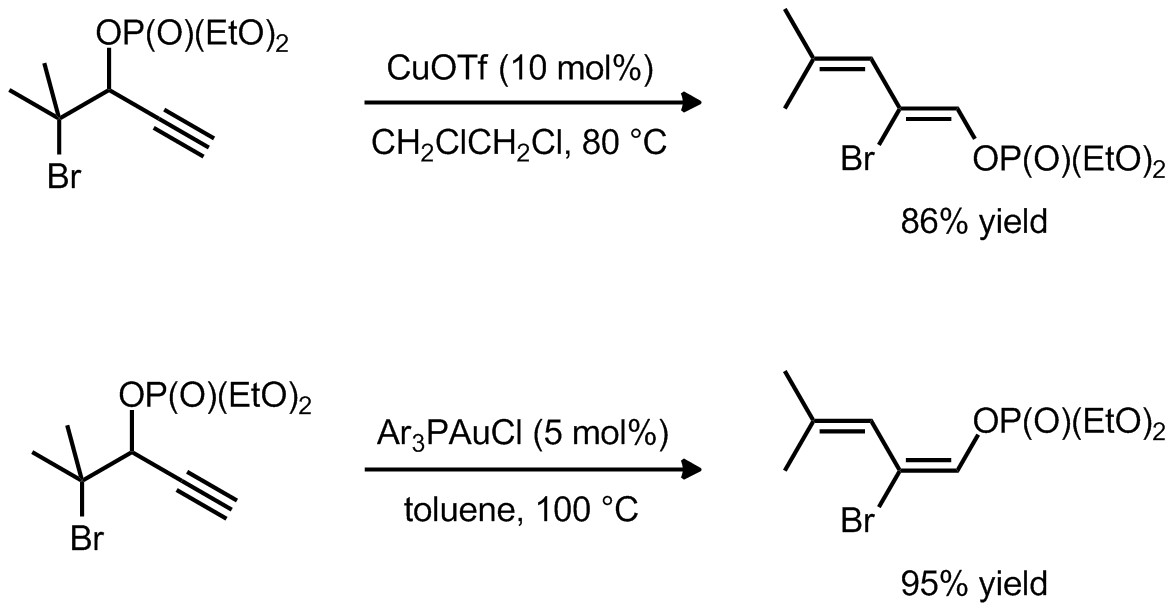
|
| Problem 57. Provide a mechanism for the following transformation. The proposed mechanism should provide an explanation for the selectivities observed in the two reactions. Contributor: G. Lalic Source: Org. Lett 2012, 14, 2940. Keywords: radical, oxidation |
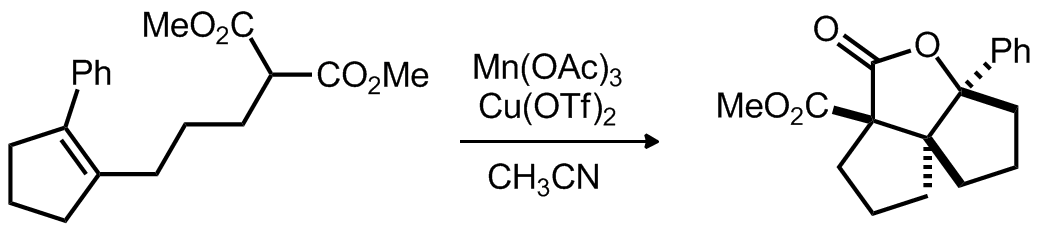
|
| Problem 56. Provide a mechanism for the following transformation. The proposed mechanism should provide an explanation for the selectivities observed in the two reactions. Contributor: G. Lalic Source: J. Am. Chem. Soc. 2012, 134, 8260. Keywords: |
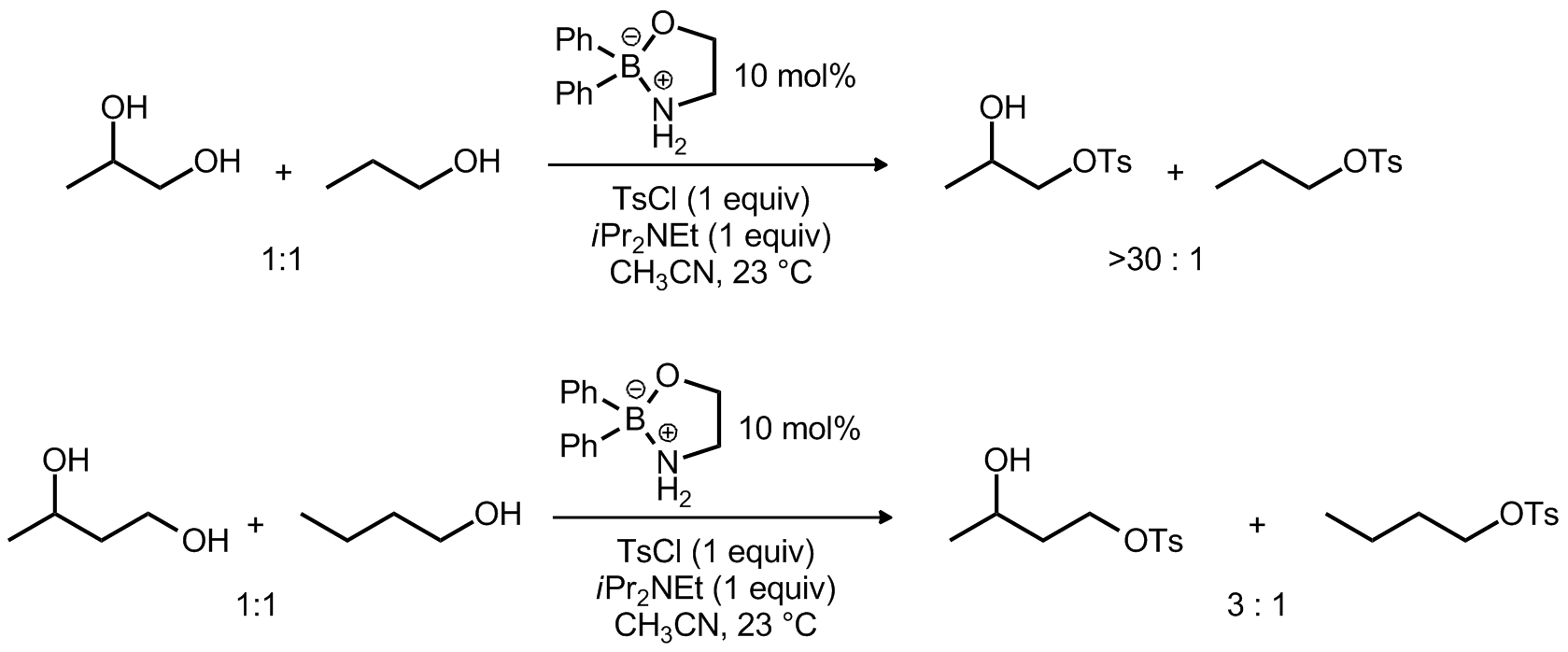
|
| Problem 55. Provide a mechanism for the following transformation. Contributor: AJ Boydston Source: Org. Lett. 2011, 13, 6034. Keywords: oxidation |
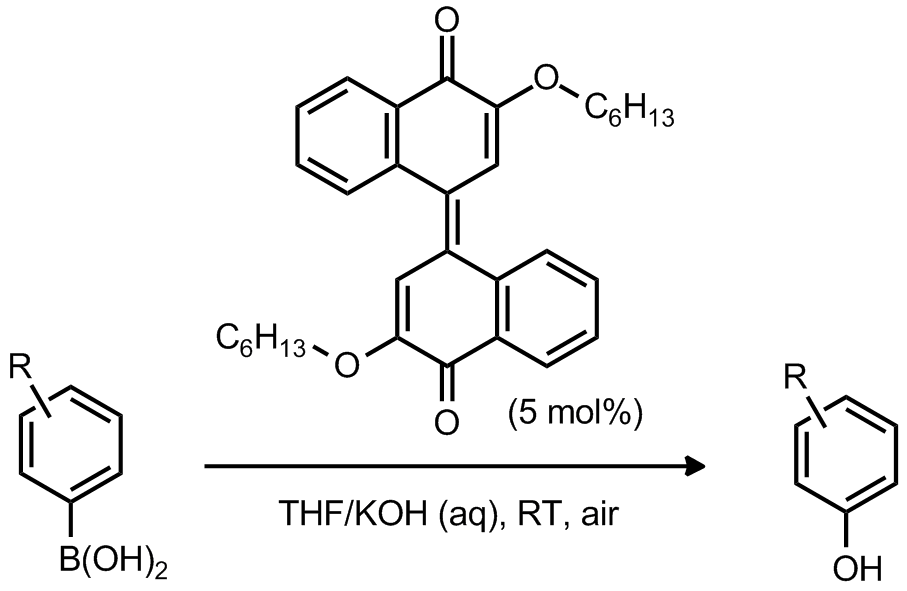
|
| Problem 54. Provide a mechanism for the following transformation. Contributor: AJ Boydston Source: J. Phys. Chem. A 2010, 114, 13086. Keywords: Knoevenagel-Doebner, decarboxylation, elimination, olefination |
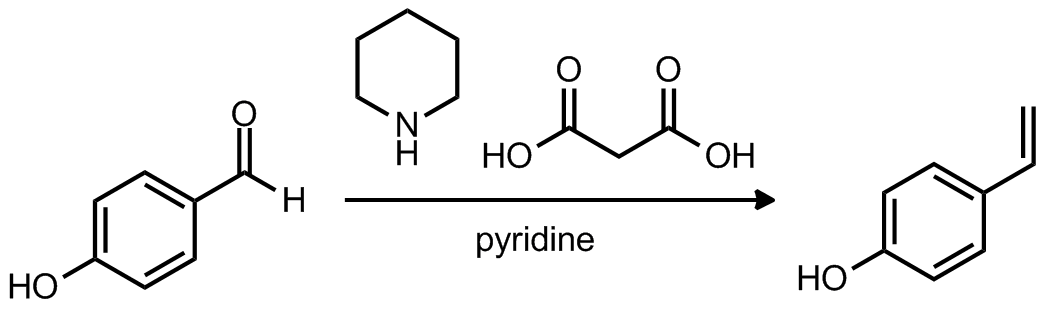
|
| Problem 53. Provide a mechanism for the following transformation. Contributor: AJ Boydston Source: Angew. Chem. Int. Ed. 2012, 51, 513. Keywords: ligation, oxaziridine, decarboxylation |
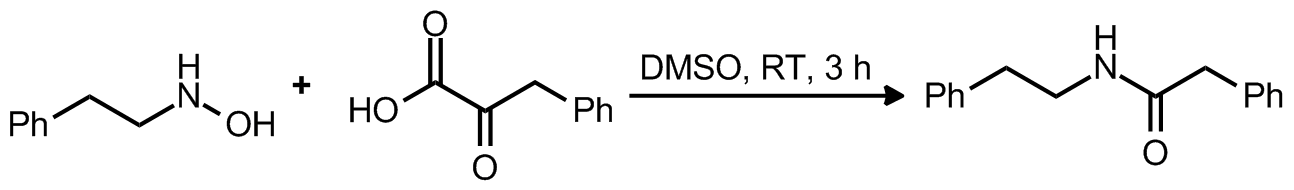
|
| Problem 52. Provide a mechanism for the following transformations. Contributor: F. Michael Source: Angew. Chem. Int. Ed. 2012, 51, 8816�8820. Keywords: radical addition |
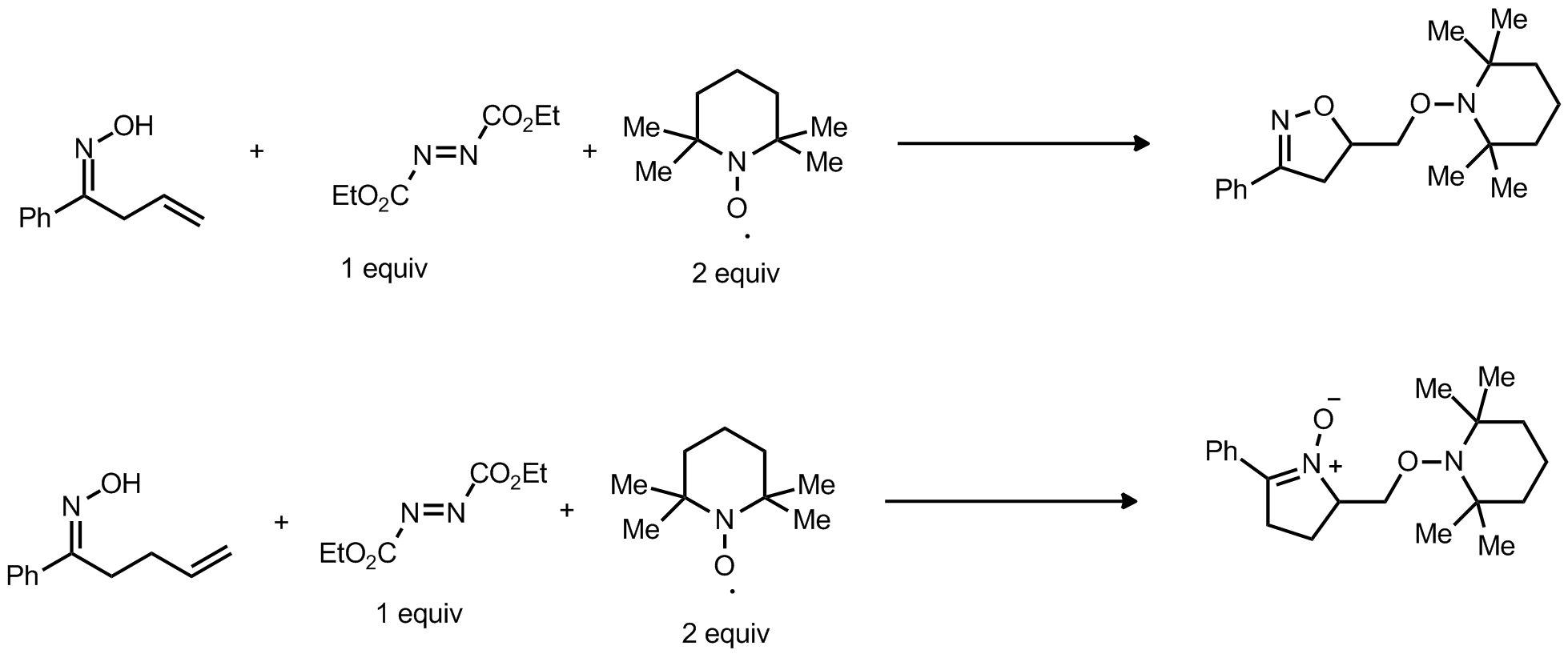
|
| Problem 51. Provide a mechanism for the following transformation. Contributor: F. Michael Source: Org. Lett., 2012, ASAP, DOI: 10.1021/ol302077n Keywords: ylide |
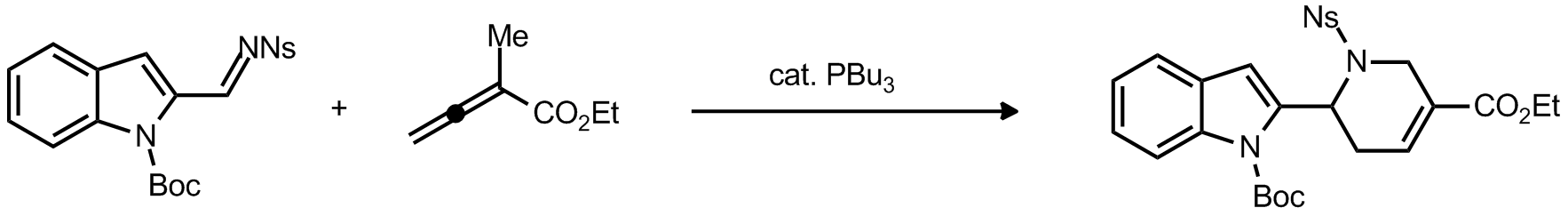
|
| Problem 50. Provide a mechanism for the following transformation. Contributor: F. Michael Source: J. Am. Chem. Soc., 2012, 134 (33), 13577�13579. Keywords: Shapiro |
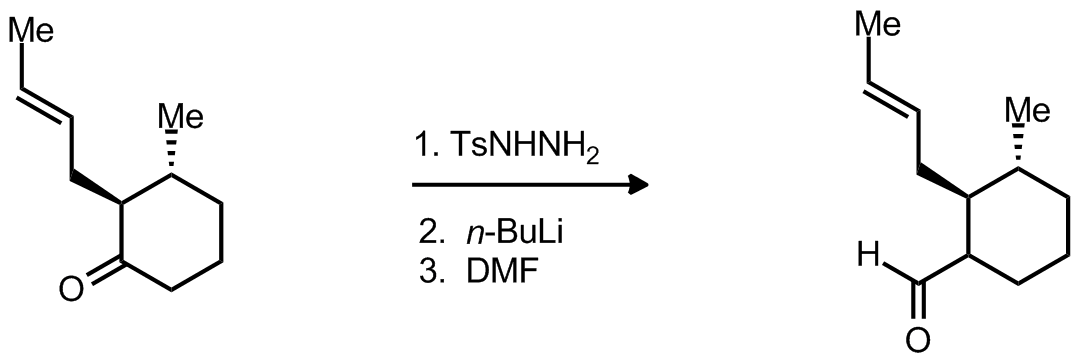
|
| Problem 49. Propose a synthesis. Contributor: F. Michael Source: J. Am. Chem. Soc., 2012, 134 (33), 13577�13579. Keywords: synthesis |
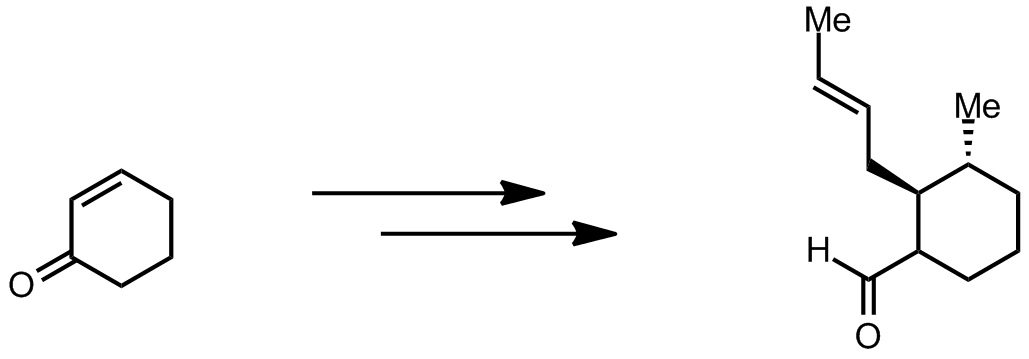
|
| Problem 48. Provide a mechanism for the following transformations. Contributor: G. Lalic Source: Org. Lett. 2012, 14, 58. Keywords: Prins |
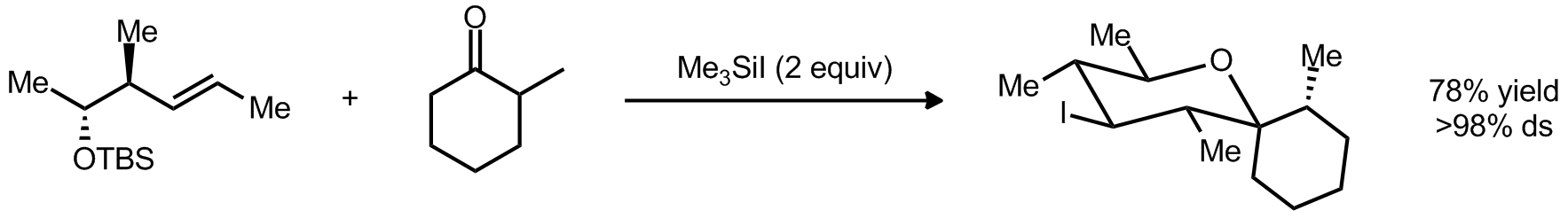
|
| Problem 47. Provide a mechanism for the following transformations. Contributor: G. Lalic Source: Org. Lett. 2012, 14, 122. Keywords: Cope rearrangement |
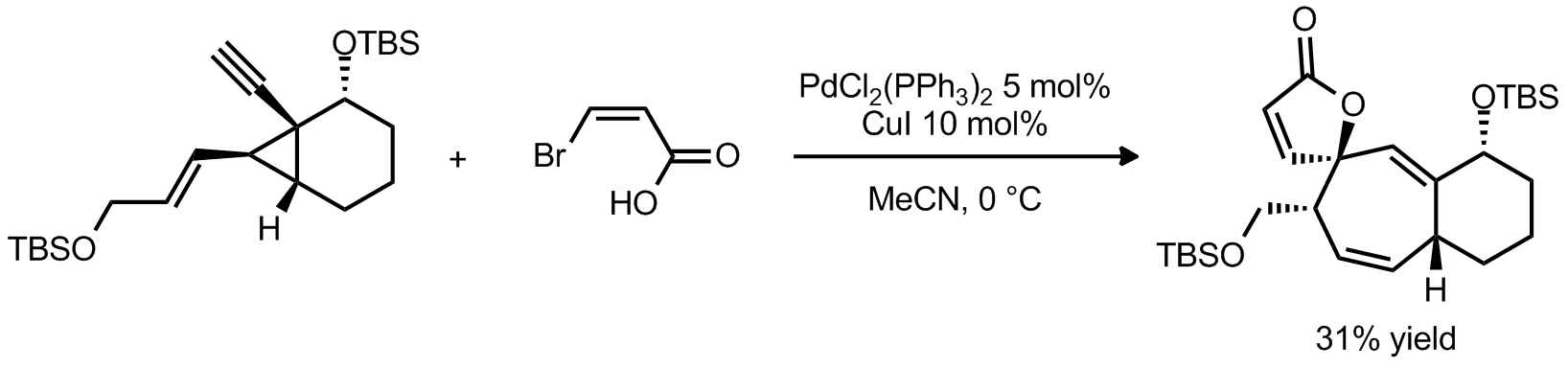
|
| Problem 46. The following is the key step in De Brabander�s synthesis of Berkelic acid. Please provide a mechanism. Contributor: G. Lalic Source: J. Am. Chem. Soc. 2009, 131, 11350. Keywords: Ortho quinone methide |
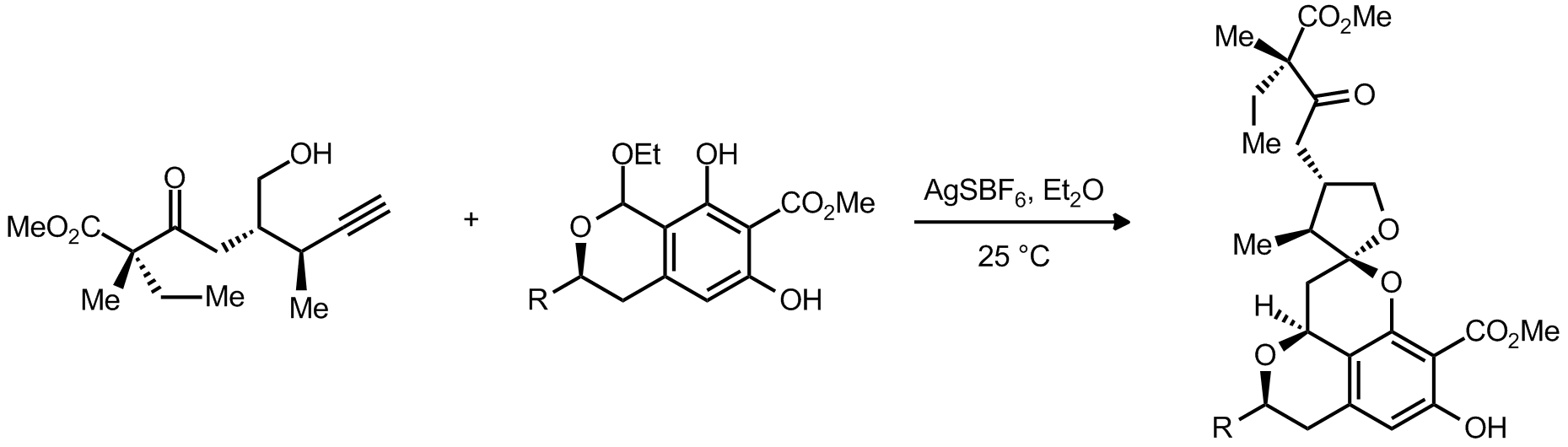
|
| Problem 45. Provide a mechanism for the following transformations. Contributor: G. Lalic Source: Org. Lett. 2012, 14, 18. Keywords: Ortho quinone methide |
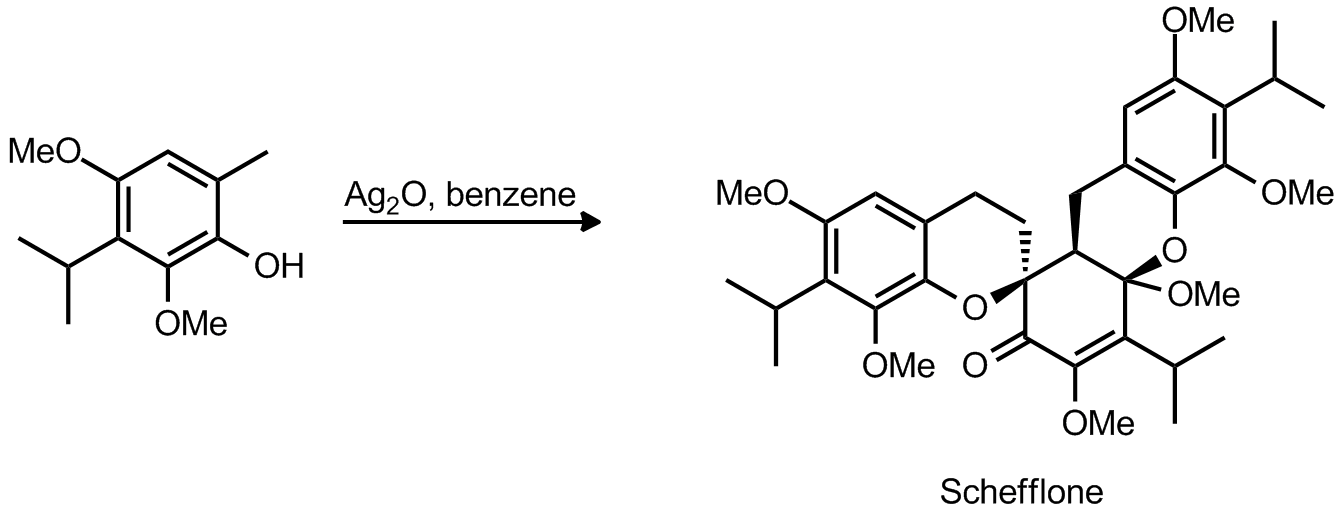
|
| Problem 44. Provide a mechanism for the following transformations. Contributor: AJ Boydston Source: J. Org. Chem. 2012(DOI:10.1021/jo300872y) Keywords: Prins, Prins-Pinacol |
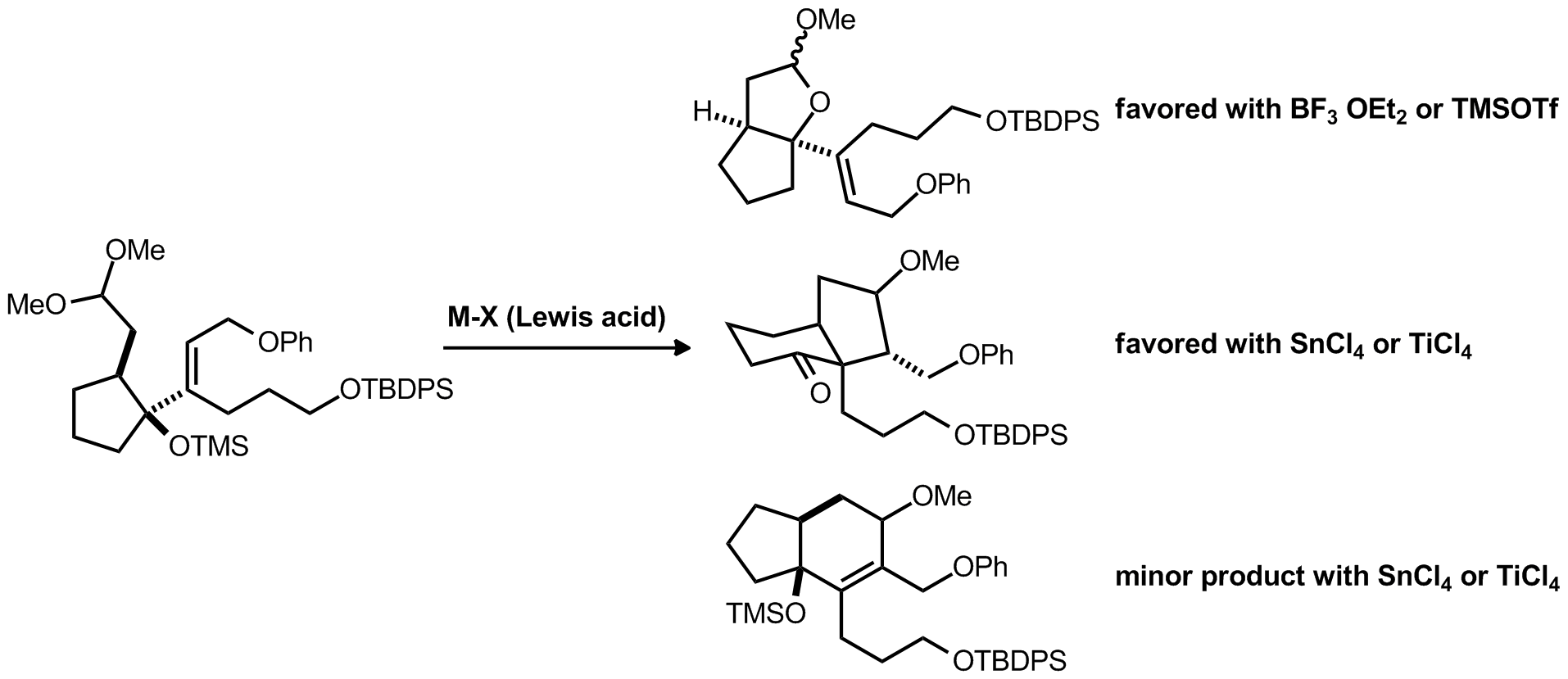
|
| Problem 43. Provide a mechanism for the following transformation and rationalize the disparate reactivity of the diastereoisomers. Contributor: AJ Boydston Source: Org. Lett. 2012, 14, 3604. Keywords: Schmidt |
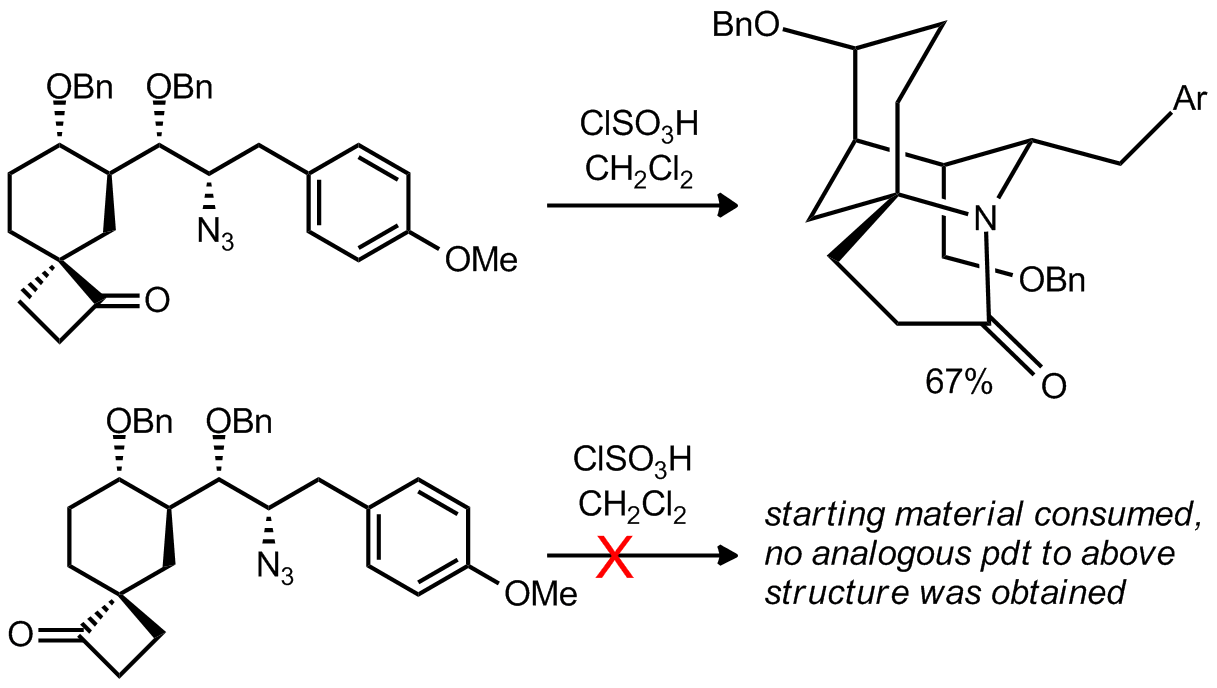
|
| Problem 42. Provide a mechanism for the following transformation. Contributor: AJ Boydston Source: Org. Lett. 2012, 14, 3604.; J. Am. Chem. Soc. 1977, 99, 7601. Keywords: epoxidation, Corey-Chaykovsky |
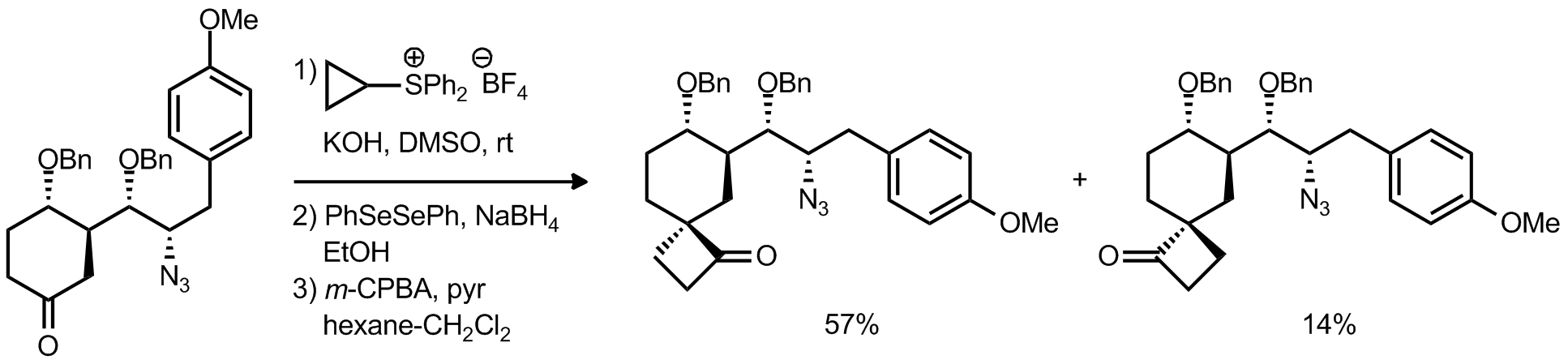
|
| Problem 41. What can be inferred about the relative rates of initiation, propagation, and termination based on the kinetic data for the shown polymerization reaction? What additional conclusions could be drawn from either braoad or narrow PDIs? Contributor: AJ Boydston Source: Synlett 2007, 10, 1573.; J. Am. Chem. Soc. 2012, 134, 9163. Keywords: polymerization |
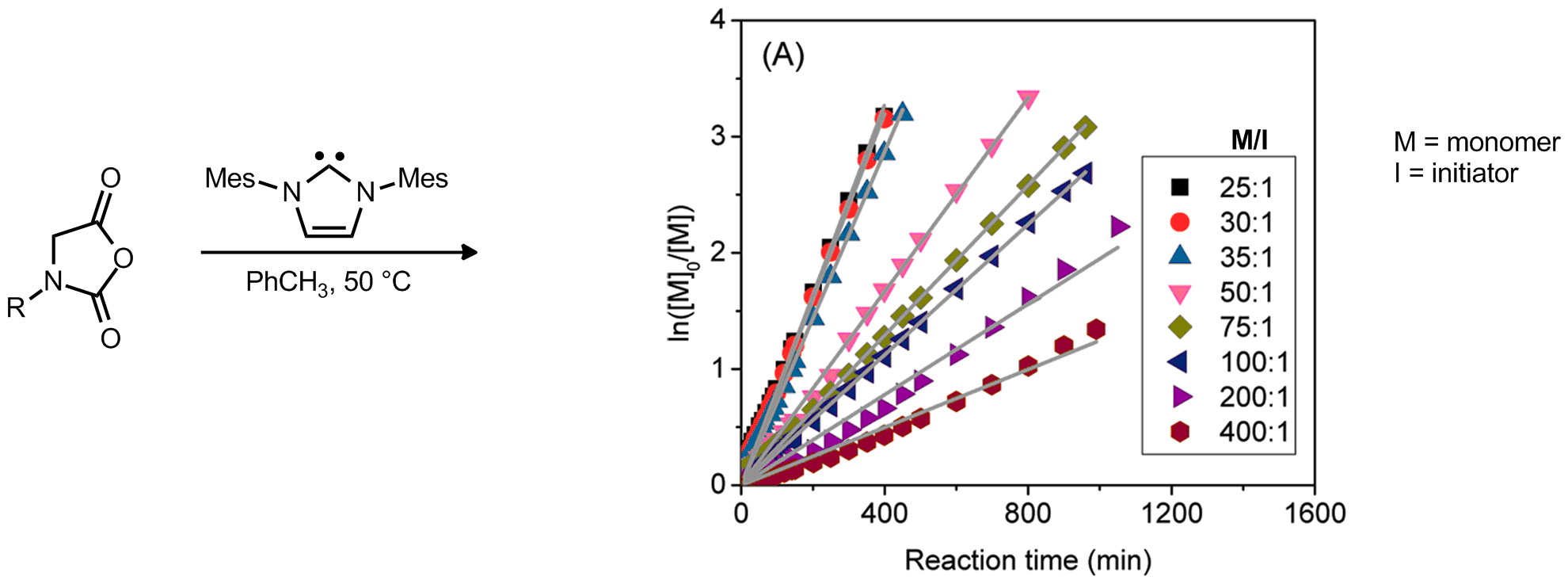
|
| Problem 40. Provide the structure of the resulting polymers. Contributor: AJ Boydston Source: Synlett 2007, 10, 1573.; J. Am. Chem. Soc. 2012, 134, 9163. Keywords: polymerization |
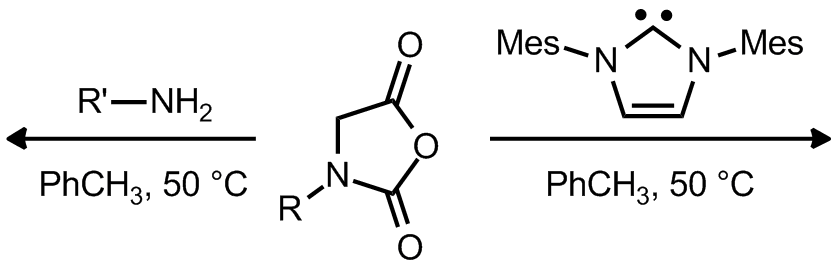
|
| Problem 39. N-Carboxyanhydrides (NCAs) are useful monomers for ring-opening polymerizations to synthesize polypeptoids. Provide a mechanism for the first step in the synthesis of the NCA. Contributor: AJ Boydston Source: Synlett 2007, 10, 1573.; J. Am. Chem. Soc. 2012, 134, 9163. Keywords: decarboxylation |
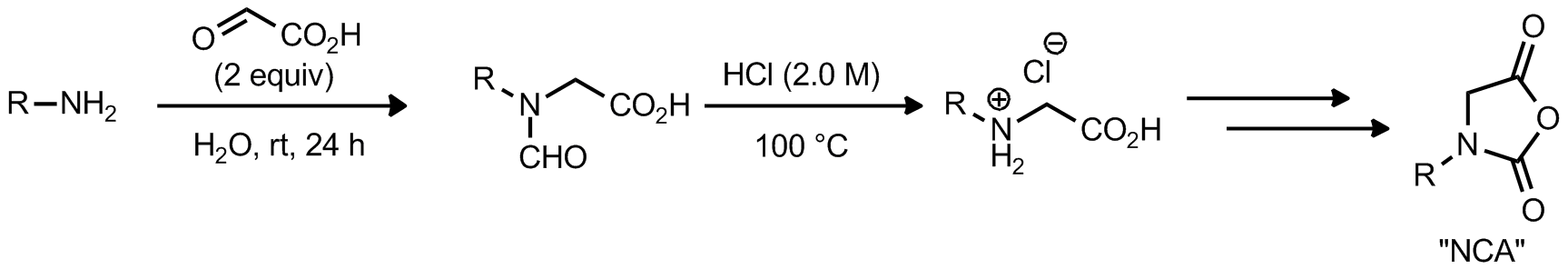
|
| Problem 38. Complete the synthetic scheme. Contributor: F. Michael Source: J. Am. Chem. Soc. 2010, 132, 15790. Keywords: cyclopropanation, allylation, Cope rearrangement |
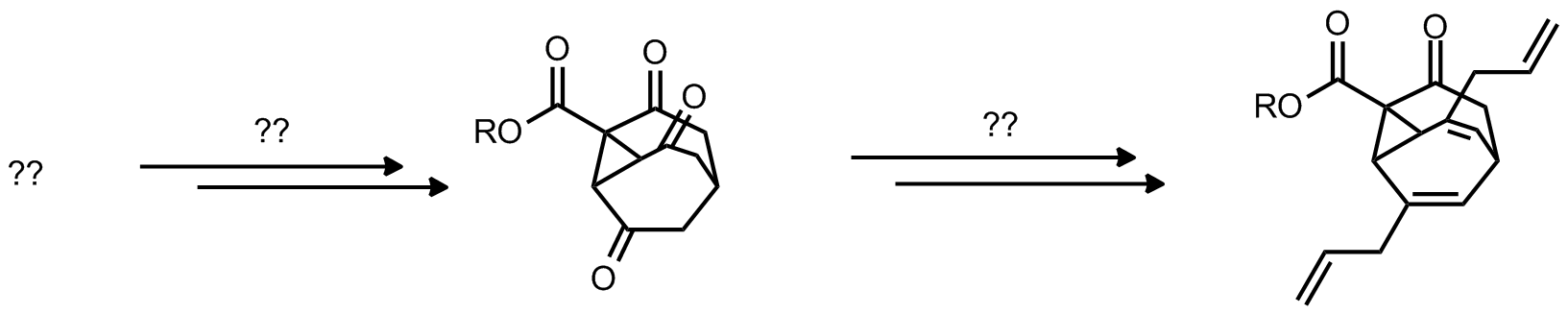
|
| Problem 37. Propose a mechanism for the following transformation. Contributor: F. Michael Source: Eur. J. Org. Chem. 2002, 791. Keywords: Diels-Alder, electrocyclic reaction |
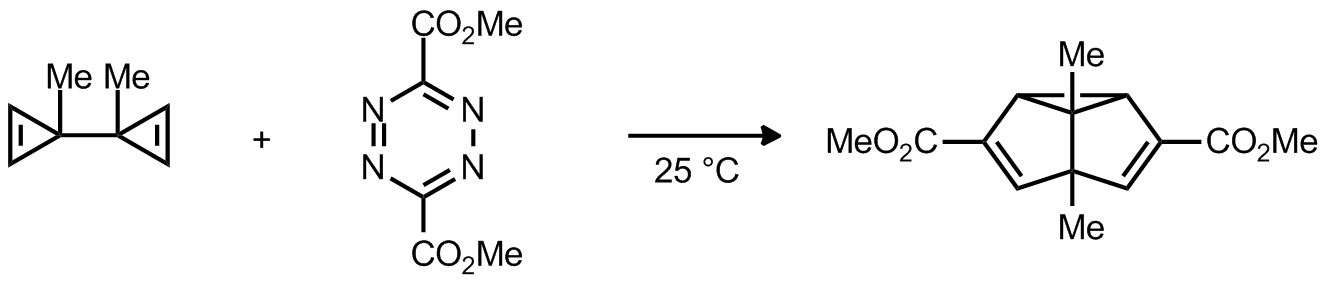
|
| Problem 36. Propose a mechanism for the foloowing transformation and identify A. Contributor: F. Michael Source: J. Am. Chem. Soc. 2012, 134, 11964. Keywords: Cope rearrangement, carbanion, Diels-Alder |
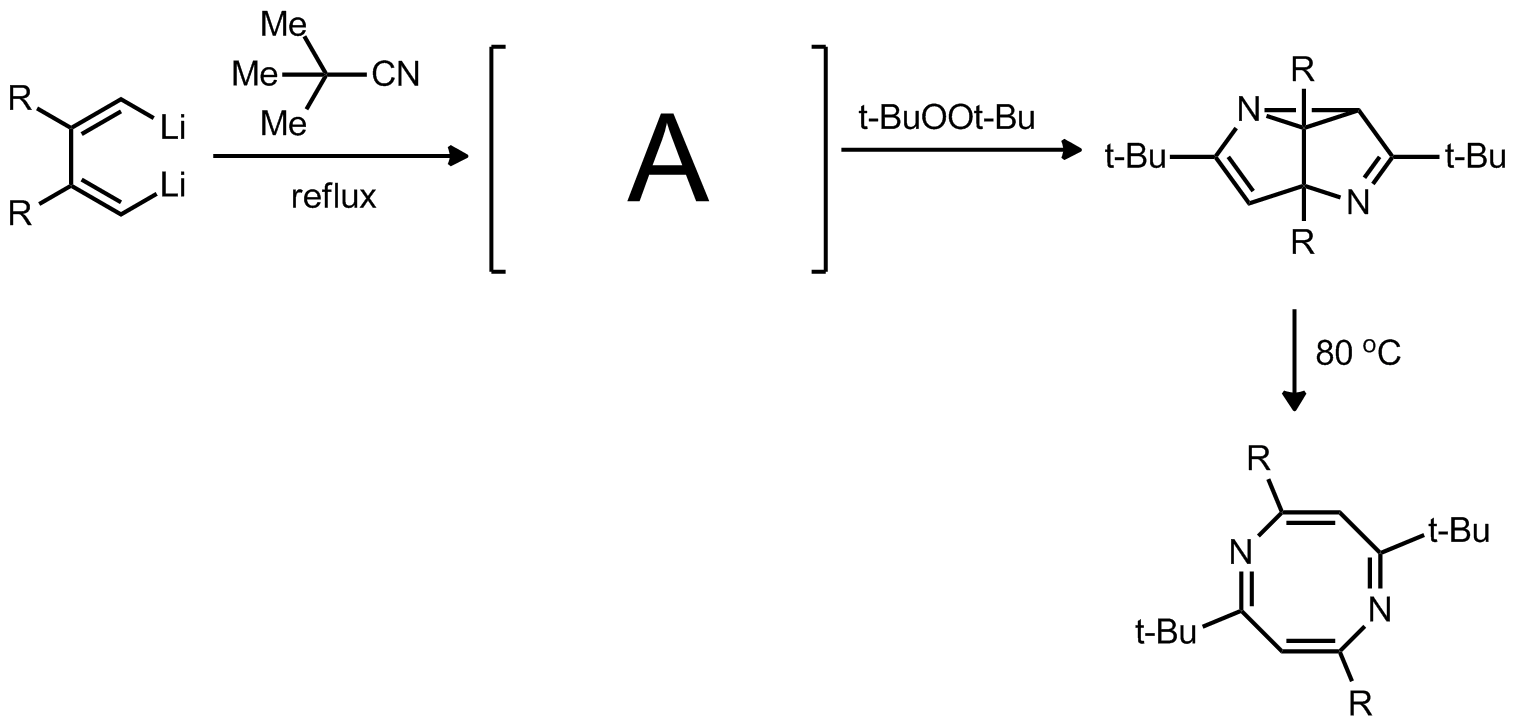
|
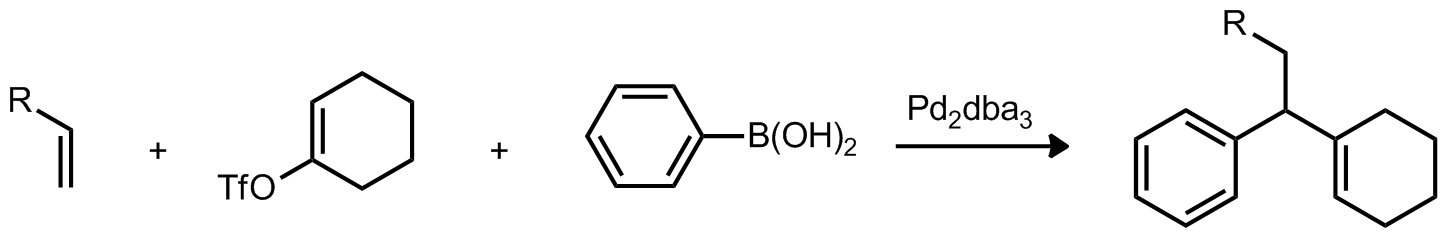
| Problem 35. Propose a mechanism for the foloowing transformation. What byproducts would you expect based on your mechanism? Contributor: F. Michael Source: J. Am. Chem. Soc. 2011, 133, 5784. Keywords: Suzuki coupling, p allyl metal complex |
|
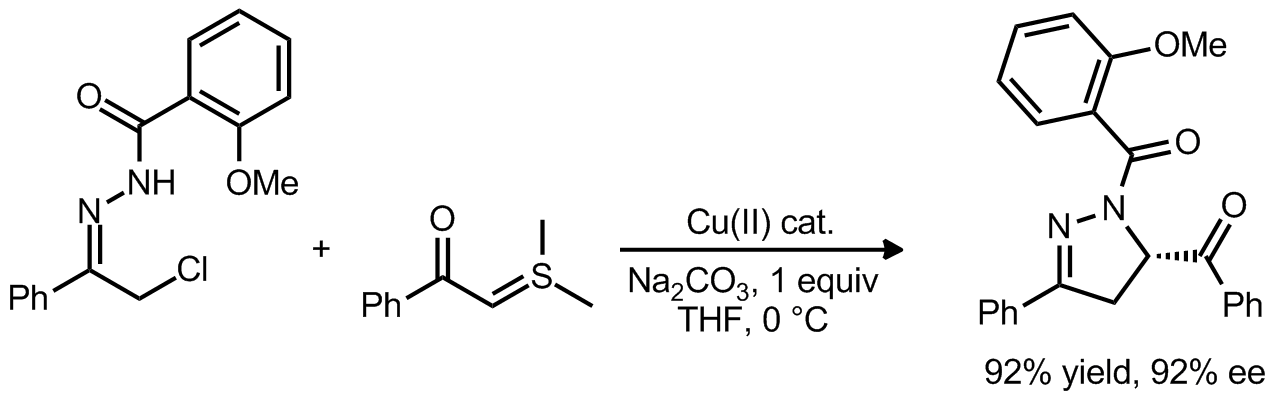
| Problem 34. Provide a mechanism for the following transformation. Contributor: G. Lalic Source: J. Am. Chem. Soc. 2012, 134, 6924. Keywords: 3+2 cycloaddition |
|
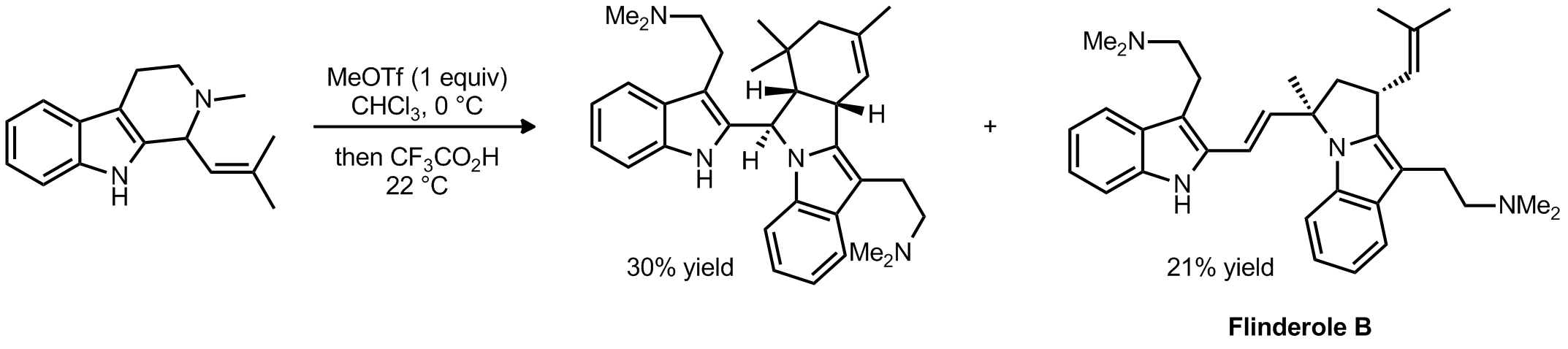
| Problem 33. In a recent total synthesis of Flinderol B, the authors reported the following transformations. Provide the mechanism for the formation of the two products. Contributor: G. Lalic Source: J. Am. Chem. Soc. 2012, 134, 6936. Keywords: Diels-Alder, 3+2 cycloaddition |
|
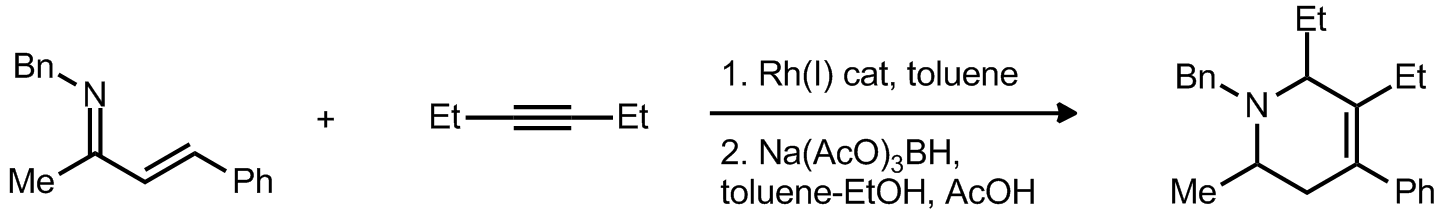
| Problem 32. Provide a mechanism for the following transformation. Contributor: G. Lalic Source: J. Am. Chem. Soc. 2012, 134, 4064 & J. Am. Chem. Soc. 2008, 130, 3645. Keywords: electrocyclic reaction, CH activation |
|
| Problem 31. Provide an explanation for the observed selectivities. Your answer should include 3D drawings of all relevant TS. Contributor: G. Lalic Source: J. Am. Chem. Soc. 2012, 134, 6528. Keywords: Curtius rearrangement |
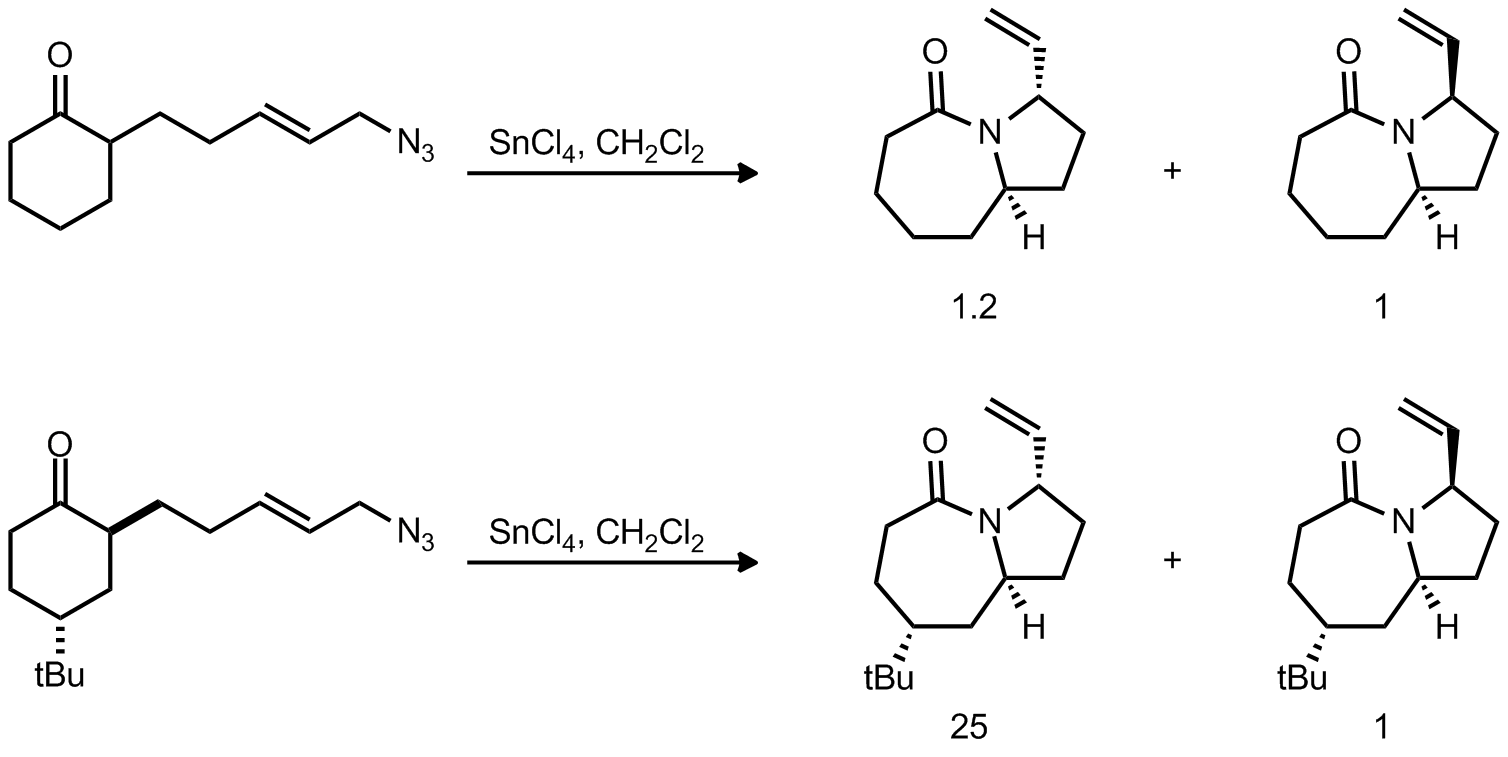
|
| Problem 30. Provide a mechanism for the following transformation. Contributor: G. Lalic Source: J. Am. Chem. Soc. 2012, 134, 6528. Keywords: Curtius rearrangement |
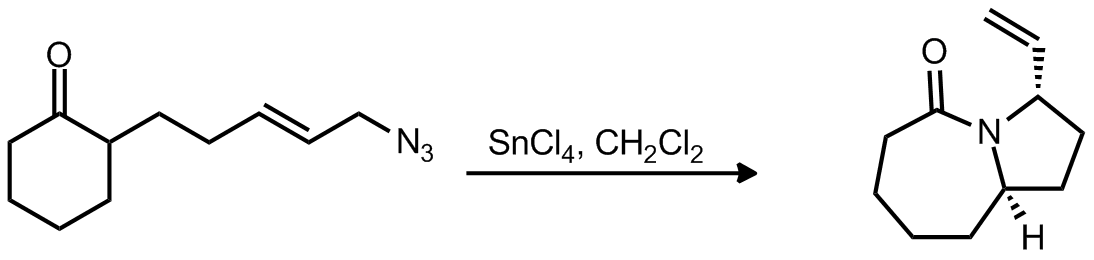
|
| Problem 29. Provide a mechanism for the following transformation. What is the role of the catalyst? What is the rate limiting step of the reaction? What is the product determining step of the reaction? Contributor: G. Lalic Source: Org. Lett. 2009, 11, 887 Keywords: Pictet�Spengler |
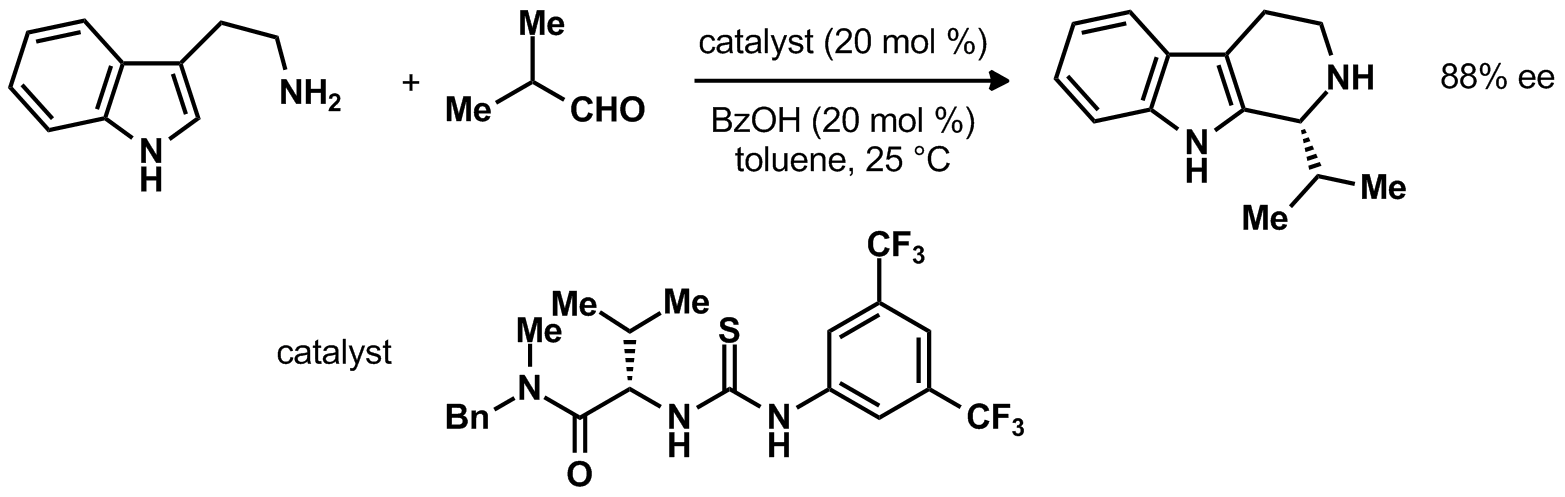
|
| Problem 28. Provide a mechanism for the following transformation. Contributor: G. Lalic Source: J. Am. Chem. Soc. 2012, 134, 1930. Keywords: metal vinylidene |
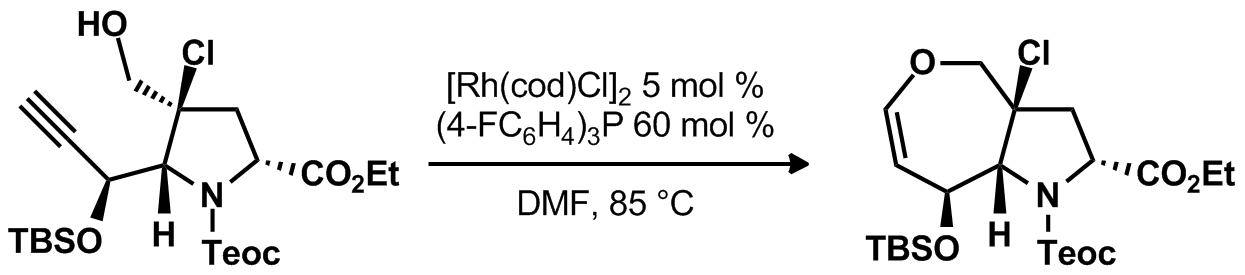
|
| Problem 27. Provide a mechanism for the following transformation. Contributor: G. Lalic Source: J. Am. Chem.Soc. 2012, 134, 2504. Keywords: hydride transfer, iminium |
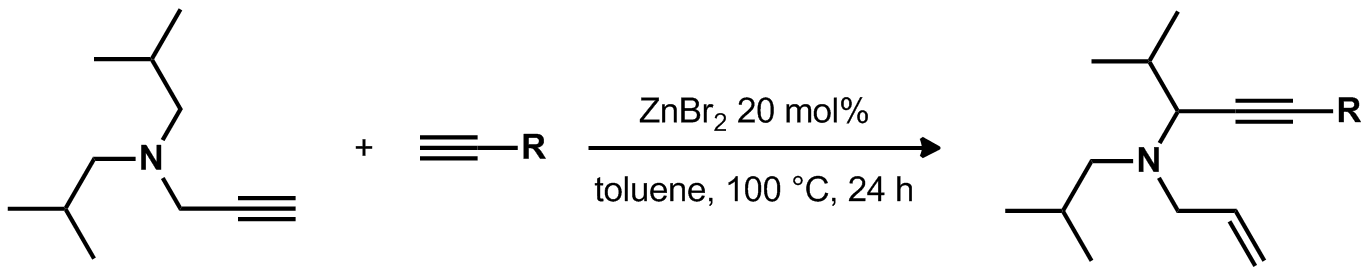
|
| Problem 26. Provide a mechanism for the following transformation. Contributor: G. Lalic Source: Angew. Chem. Int. Ed. 2011, 50, 5932. Keywords: carbene, metal carbenoid, cyclopropanation |
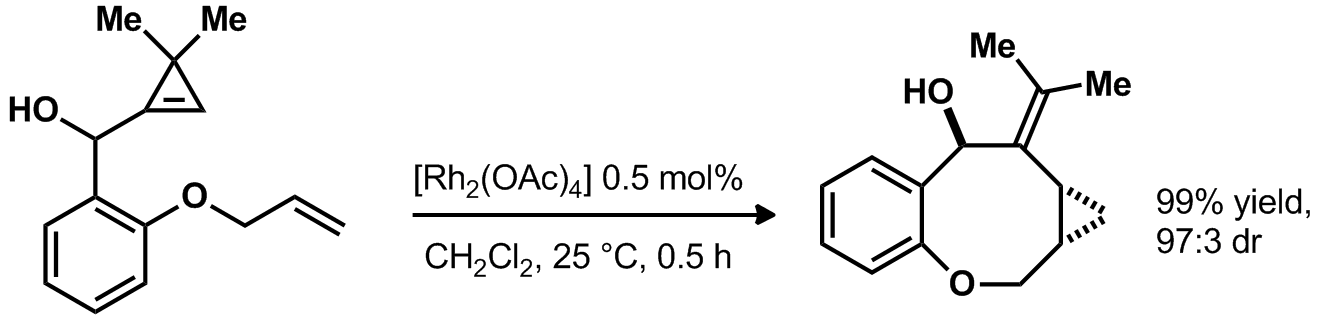
|
| Problem 25. Based on information presented in reactions A and B, provide a mechanism for transformation C. Contributor: AJ Boydston Source: Org. Lett. 2011, 13, 1678. and J. Am. Chem. Soc. 2002, 124, 9368. Keywords: electrochemistry |
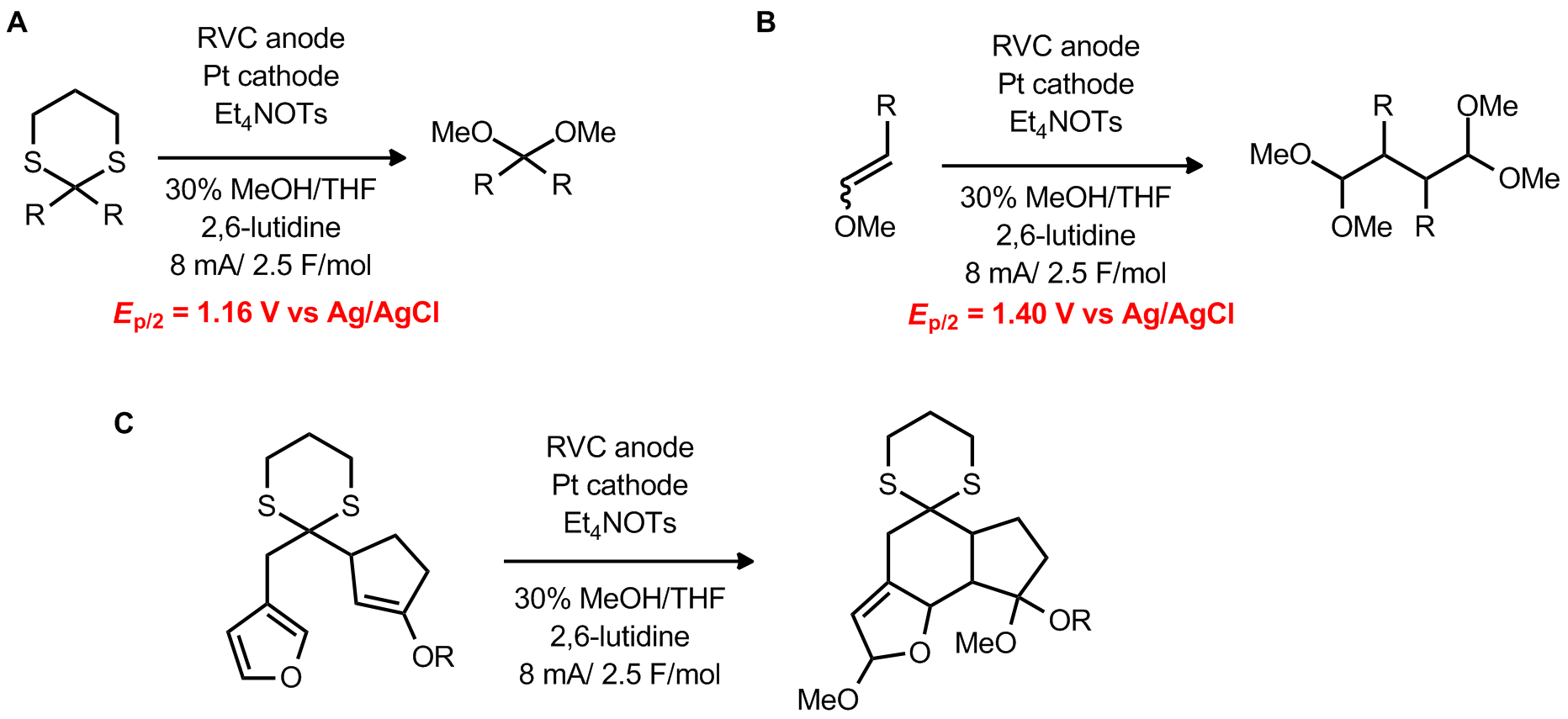
|
| Problem 24. Provide a mechanism for the following transformations. Provide the structure of product A. Contributor: AJ Boydston Source: J. Am. Chem. Soc. ASAP (10.1021/ja3030824) Keywords: CH activation |
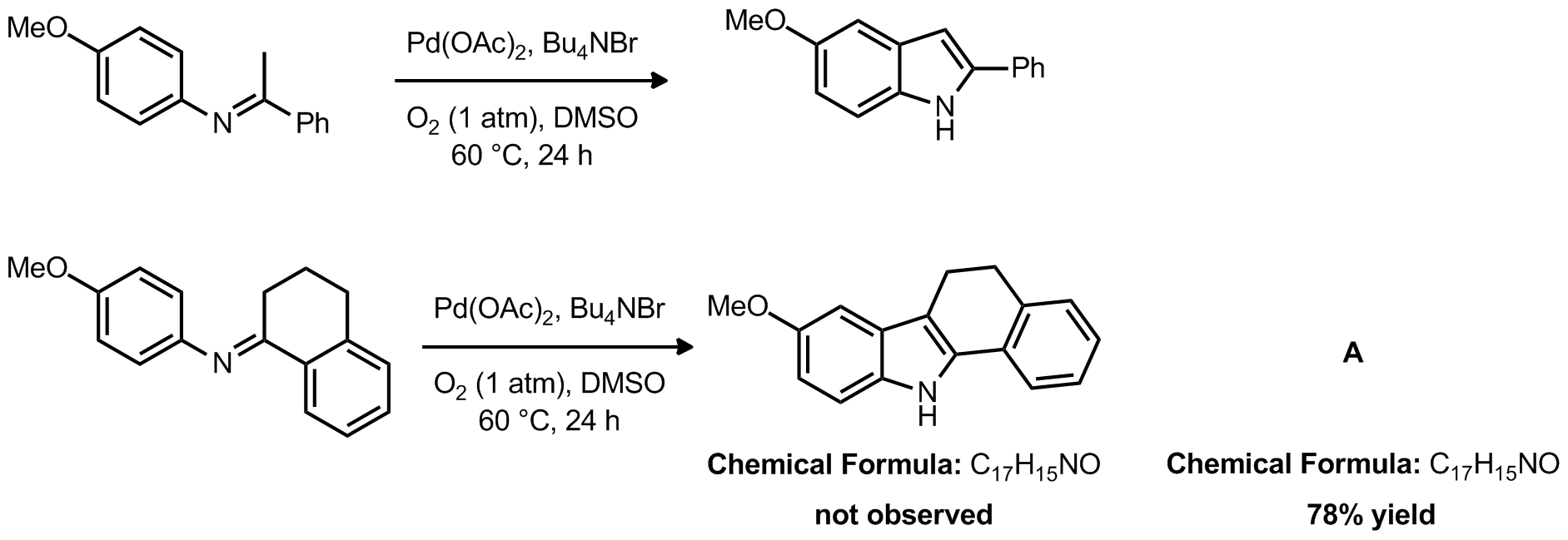
|
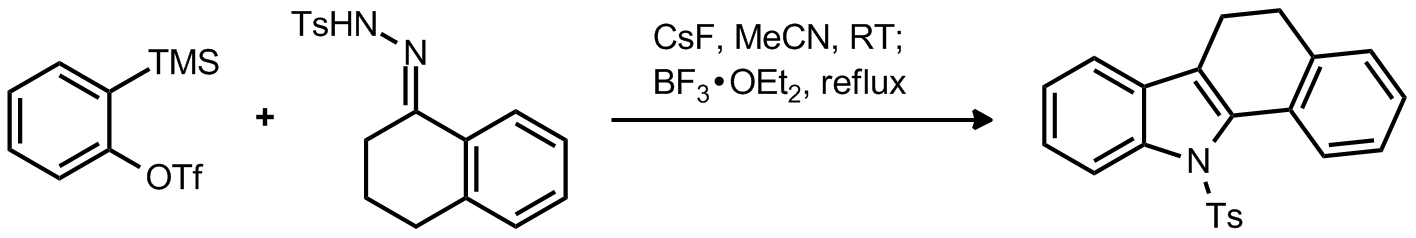
| Problem 23. Provide a mechanism for the following transformation. Contributor: AJ Boydston Source: Org. Lett. 2011, 13, 3667. Keywords: Fischer Indol |
|
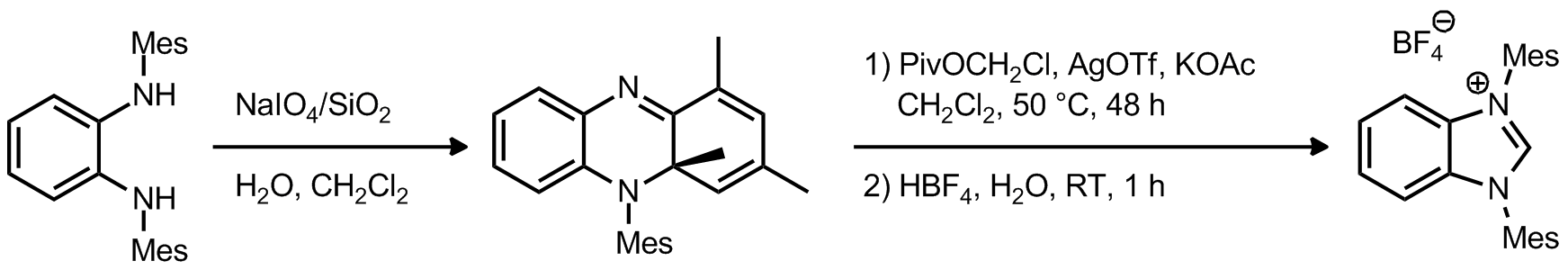
| Problem 22. Provide a mechanism for the following transformation. Contributor: AJ Boydston Source: Adv. Synth. Catal. 2012, 354, 1356. Keywords: electrocyclic reaction, sigmatropic rearrangement |
|
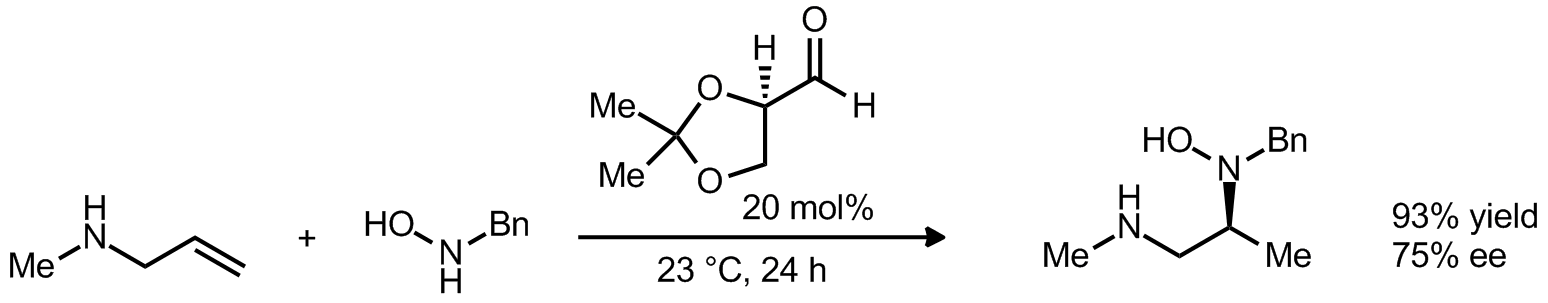
| Problem 21. Provide a mechanism for the following transformation. Contributor: G Lalic Source: J. Am. Chem. Soc. 2011, 133, 20100. Keywords: Cope elimination |
|
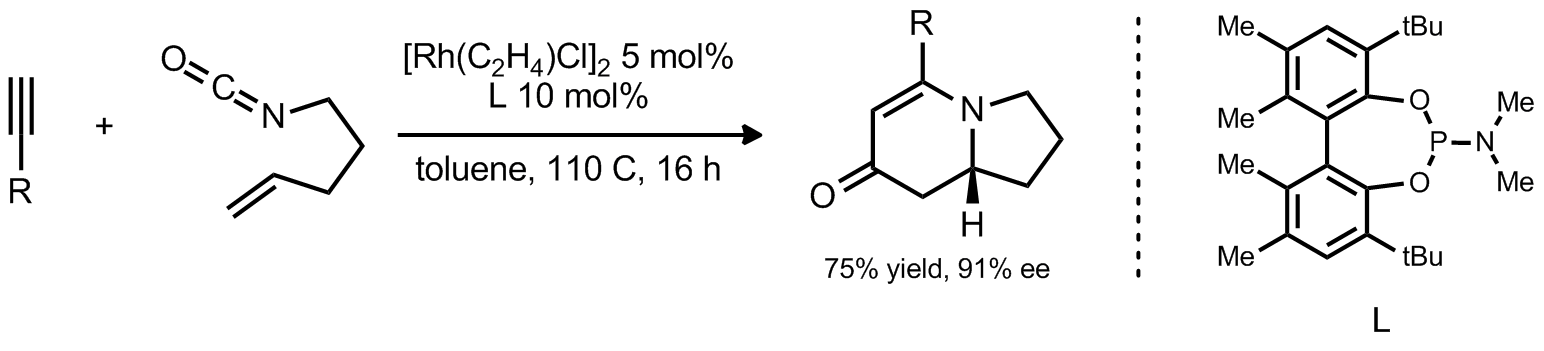
| Problem 20. Provide a mechanism for the following transformation. Contributor: G Lalic Source: Pure Appl. Chem. 2010, 82, 1353. Keywords: cycloisomerization |
|
| Problem 19. Provide a mechanism for the following transformation. Contributor: G Lalic Source: Pure Appl. Chem. 2010, 82, 1353. Keywords: cycloisomerization |
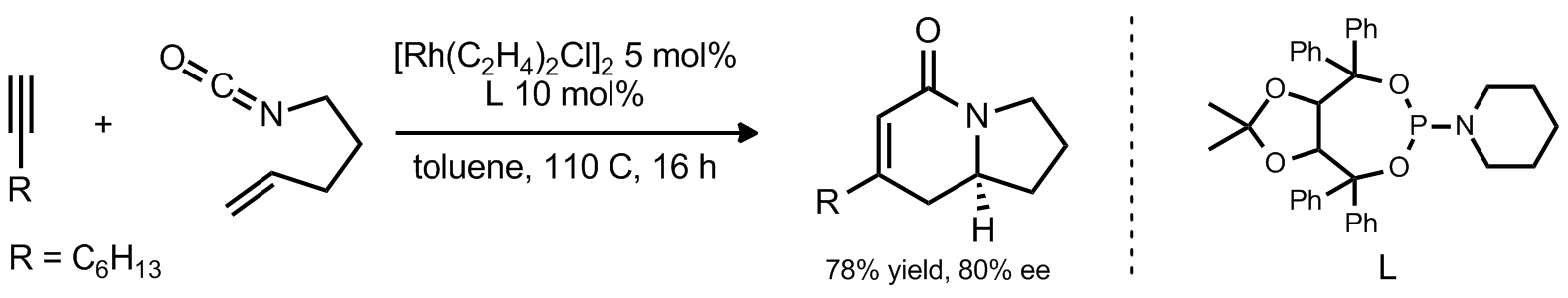
|
| Problem 18. Provide a mechanism for the following transformation and the structure of A. Contributor: G Lalic Source: Chem. Commun. 2007, 2633. Keywords: Pauson-Khand, decarbonylation |
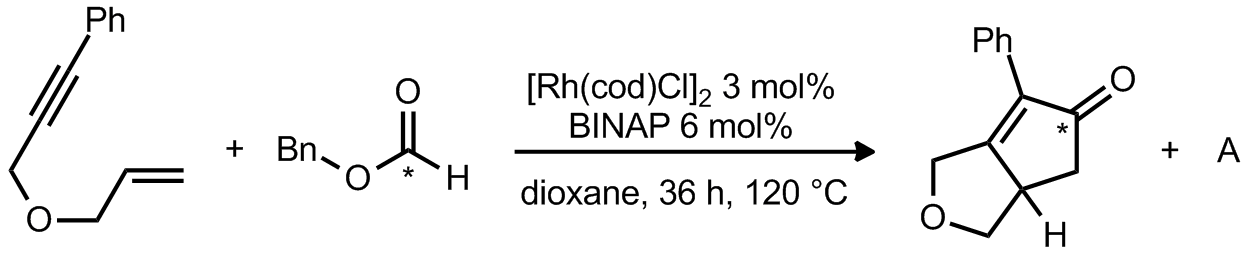
|
| Problem 17. Provide a mechanism for the following transformation. Contributor: G Lalic Source: J. Am. Chem. Soc. 2007, 129, 1874. Keywords: cycloisomerization |
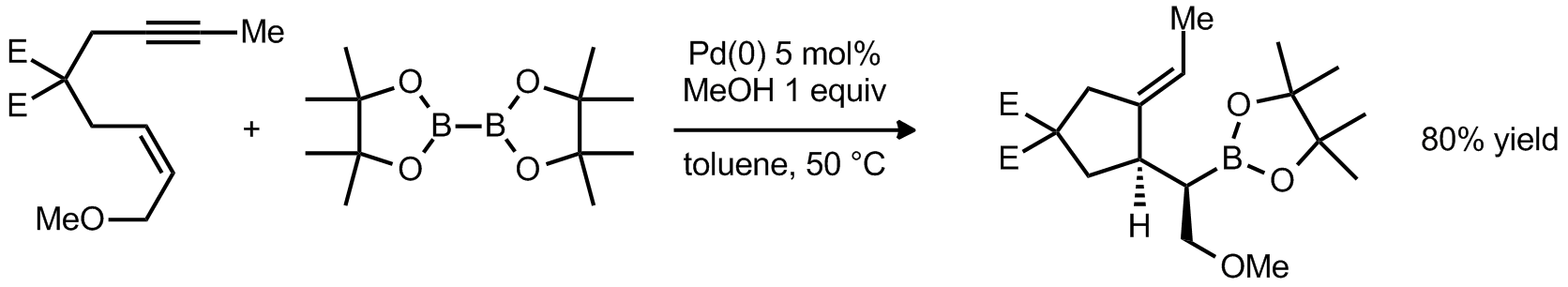
|
| Problem 16. Provide a mechanism for the following transformation. Contributor: G Lalic Source: J. Am. Chem. Soc. 1994, 116, 4268. Keywords: cycloisomerization |
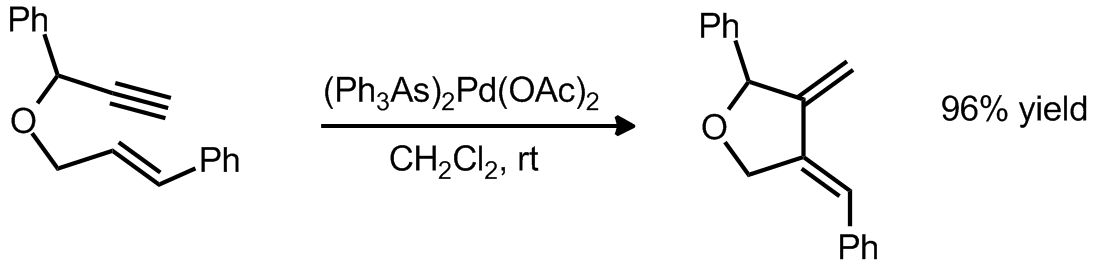
|
| Problem 15. Provide a mechanism for the following transformation. Contributor: F Michael Source: J. Am. Chem. Soc. 2012, 134, 6936. Keywords: Pictet�Spengler, iminium |
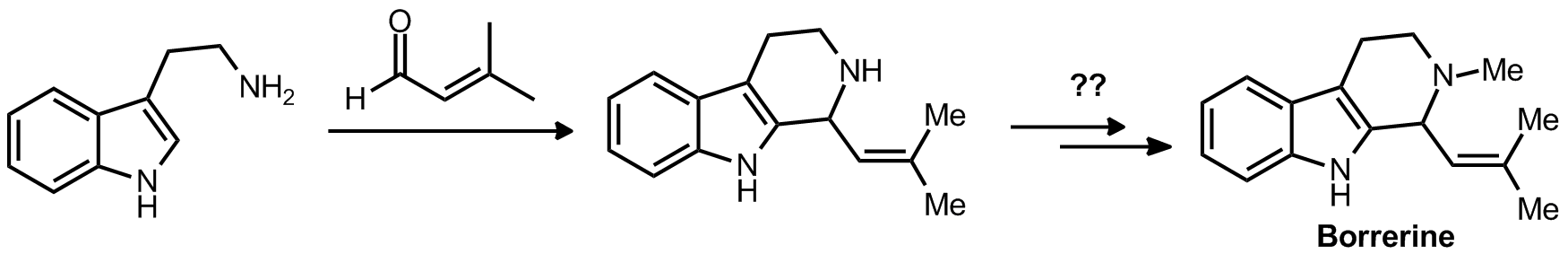
|
| Problem 14. Provide a mechanism for the following transformation. Contributor: F Michael Source: J. Am. Chem. Soc. 2009, 131, 8798. Keywords: acylketene |
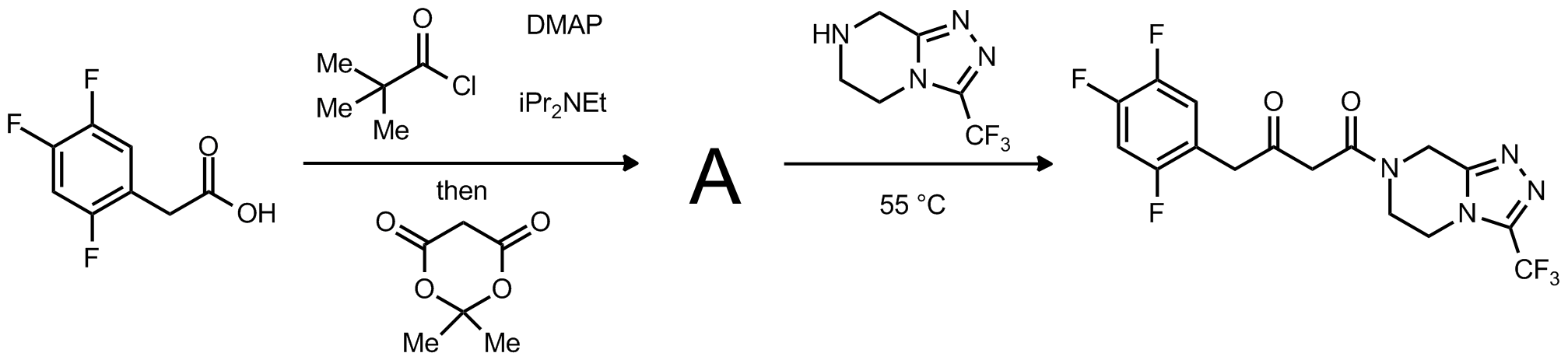
|
| Problem 13. Provide a mechanism for the following transformation. Explain the stereochemistry. Contributor: F Michael Source: Org. Lett. 2011, 13, 1490. Keywords: rearrangement |
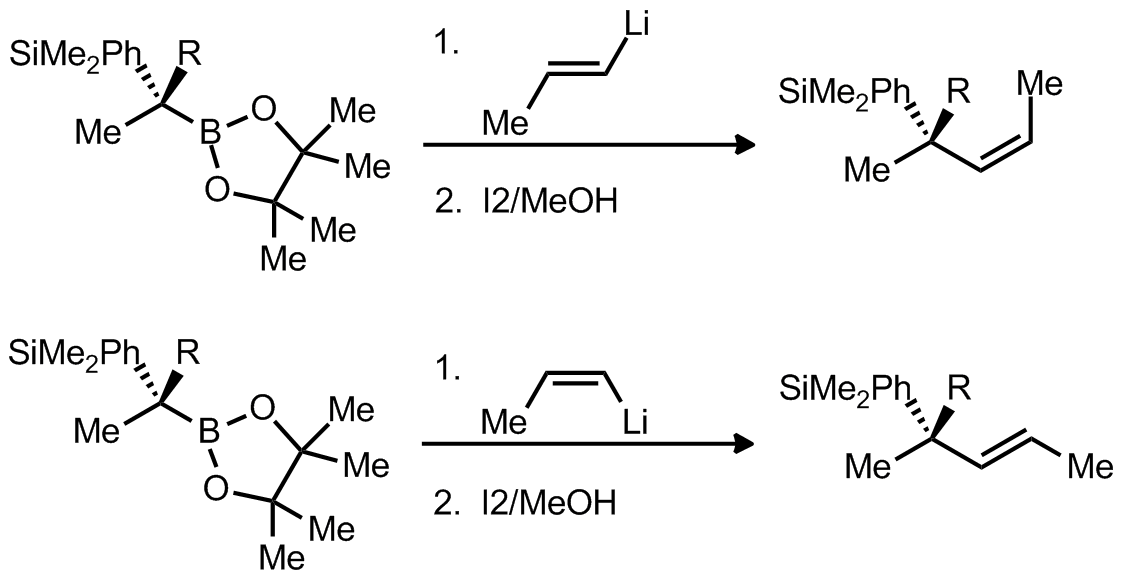
|
| Problem 12. Provide a mechanism for the following transformation. Explain the stereochemistry. Contributor: F Michael Source: J. Am. Chem. Soc. 2012, 134, 7570. Keywords: rearrangement |
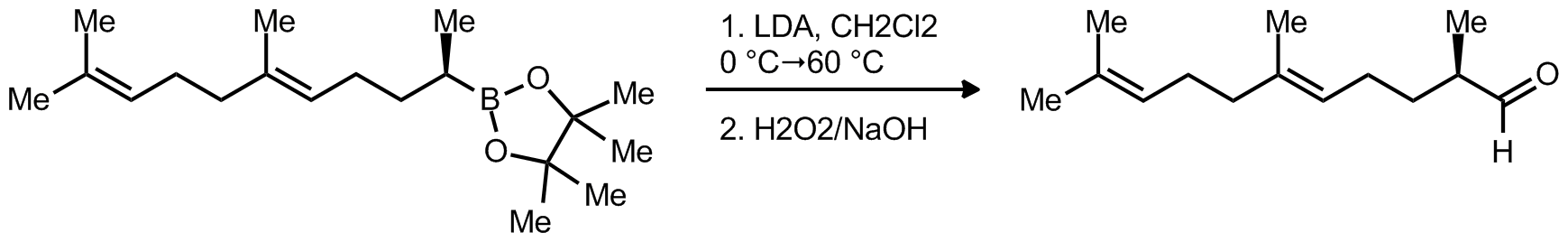
|
| Problem 11. Provide a mechanism for the following transformation. Explain the stereochemistry. Contributor: F Michael Source: Org. Lett. 2011, 13, 1490. Keywords: rearrangement |
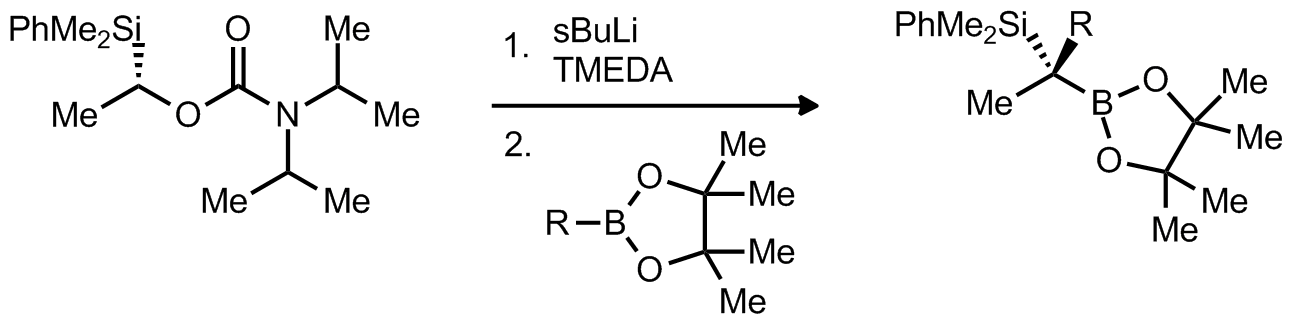
|
| Problem 10. Provide a mechanism for the following transformation Contributor: AJ Boydston Source: J. Am. Chem. Soc. 1997, 119, 2965. Keywords: radical |
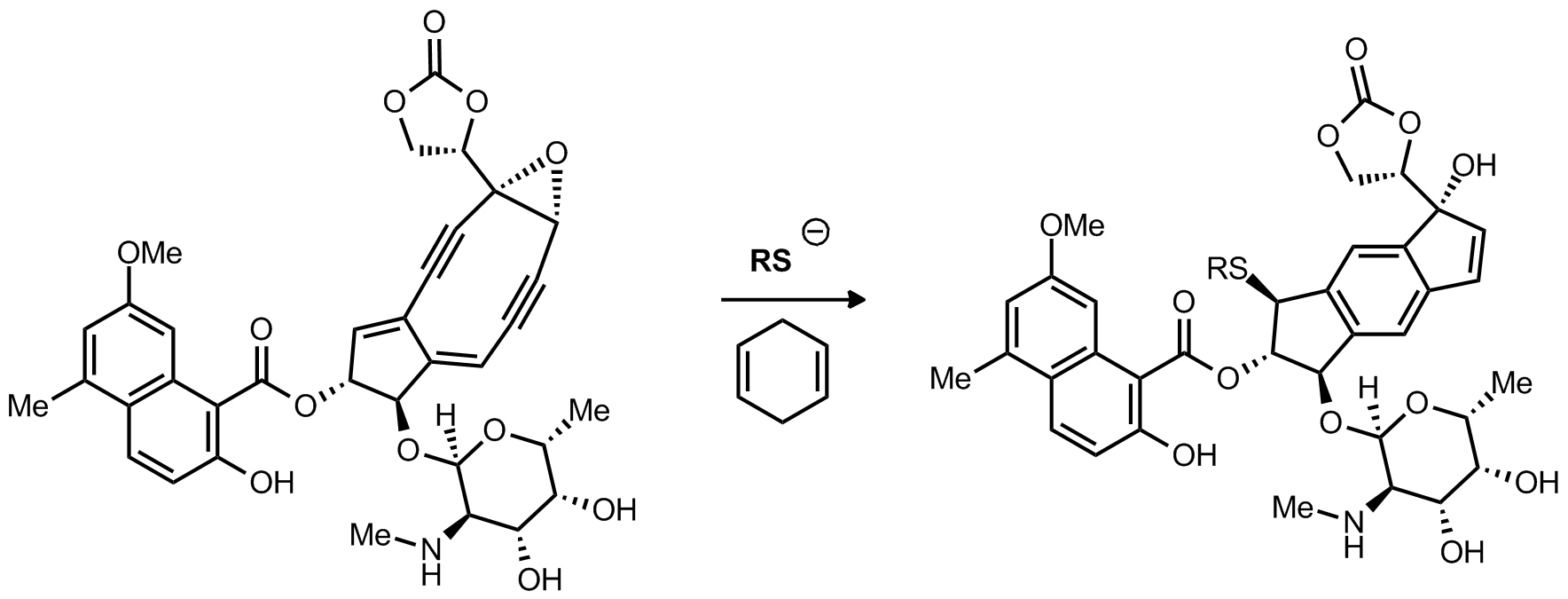
|
| Problem 9. Provide a mechanism for the following transformation Contributor: AJ Boydston Source: J. Org. Chem. 1988, 53, 3351. Keywords: cycloaddition |
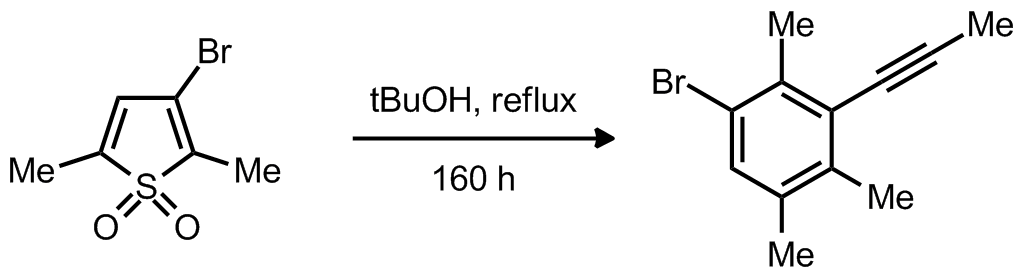
|
| Problem 8. Provide a mechanism for the following transformation Contributor: AJ Boydston Source: Org. Lett. 2011, 13, 4790. Keywords: organocatalysis |
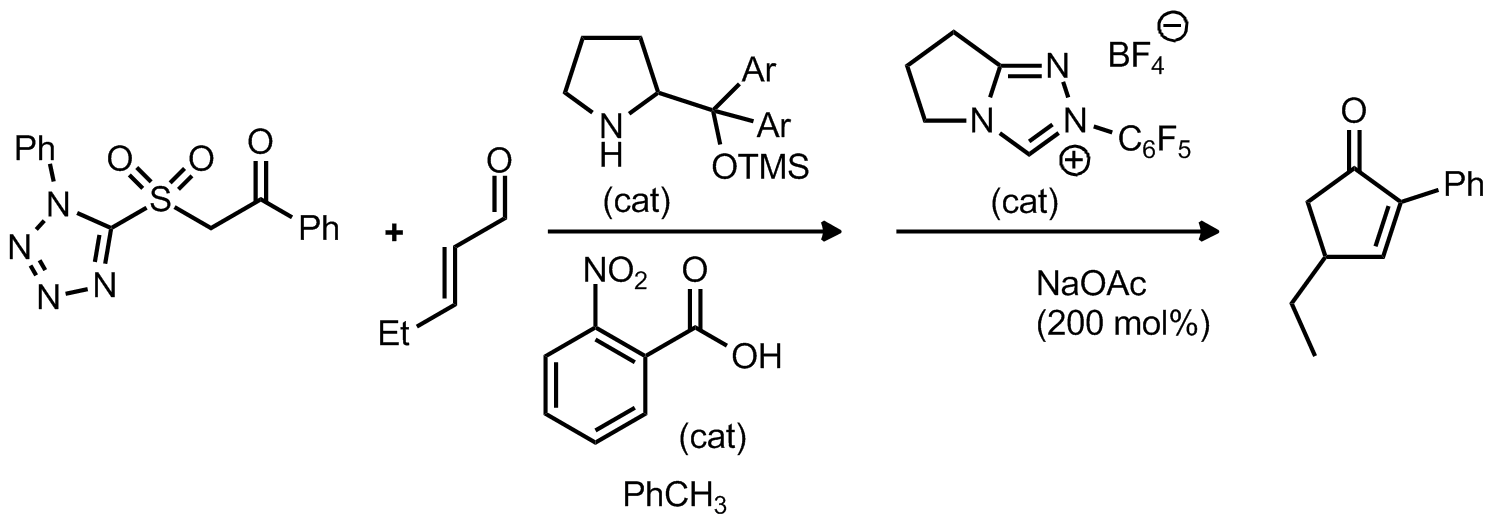
|
| Problem 7. Provide a mechanism for the following transformation Contributor: AJ Boydston Source: Angew. Chem. Int. Ed. 2012, 51, 4288. Keywords: iminium, sigmatropic rearrangement |
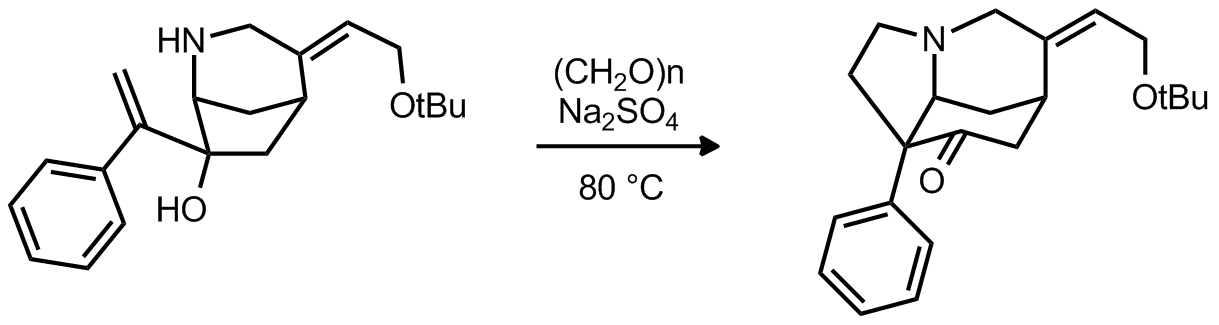
|
| Problem 6. Provide a mechanism for the following transformation Contributor: G Lalic Source: Organometallics. 2002, 21, 3845. Keywords: metal vinylidene |
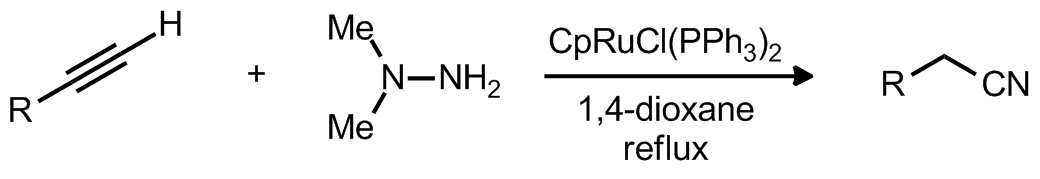
|
| Problem 5. Provide a mechanism for the following transformation Contributor: G Lalic Source: Angew. Chem. Int. Ed. 2011, 50, 5892. Keywords: Prins, oxonium |
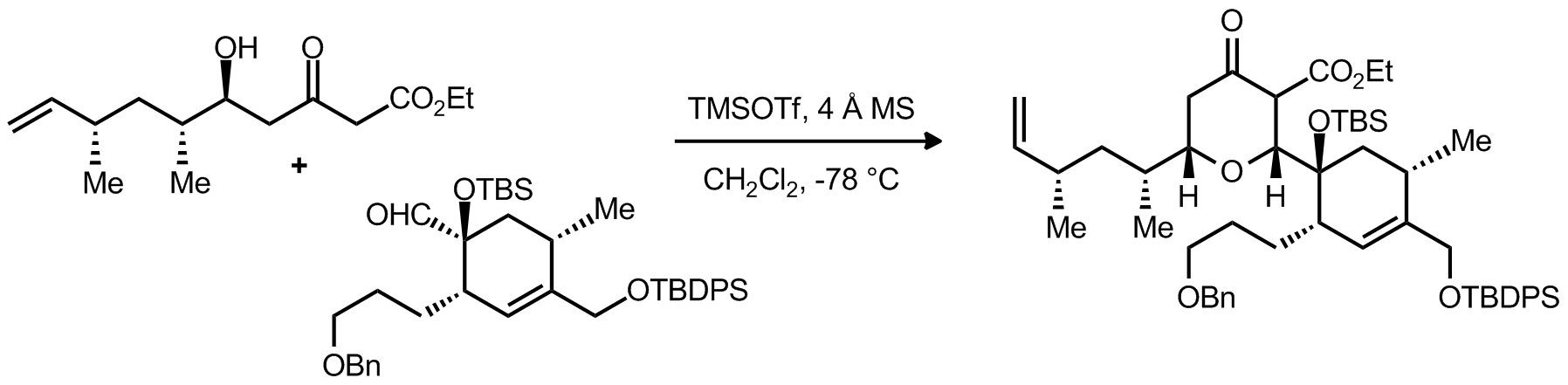
|
| Problem 4. Provide a mechanism for the following transformation Contributor: G Lalic Source: Chem. Rev. 2004, 104, 2401. Keywords: Pummerer rearrangement, oxonium |
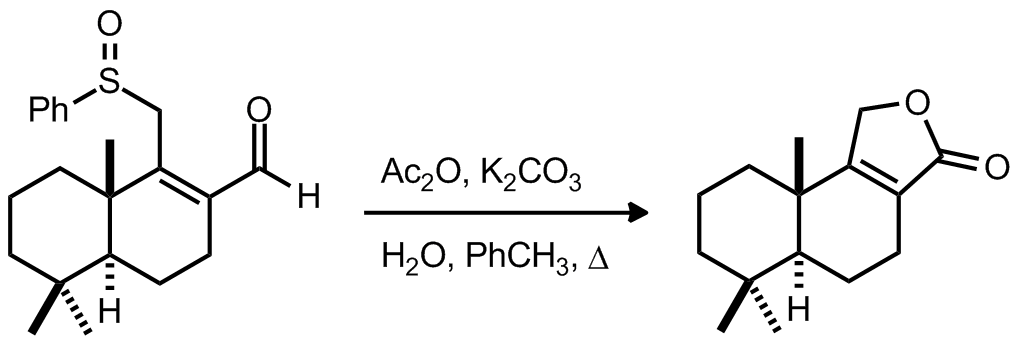
|
| Problem 3. Provide a mechanism for the following transformation Contributor: AJ Boydston Source: Tetrahedron Lett. 1993, 34, 439. Keywords: electrophilic aromatic substitution, racemization |
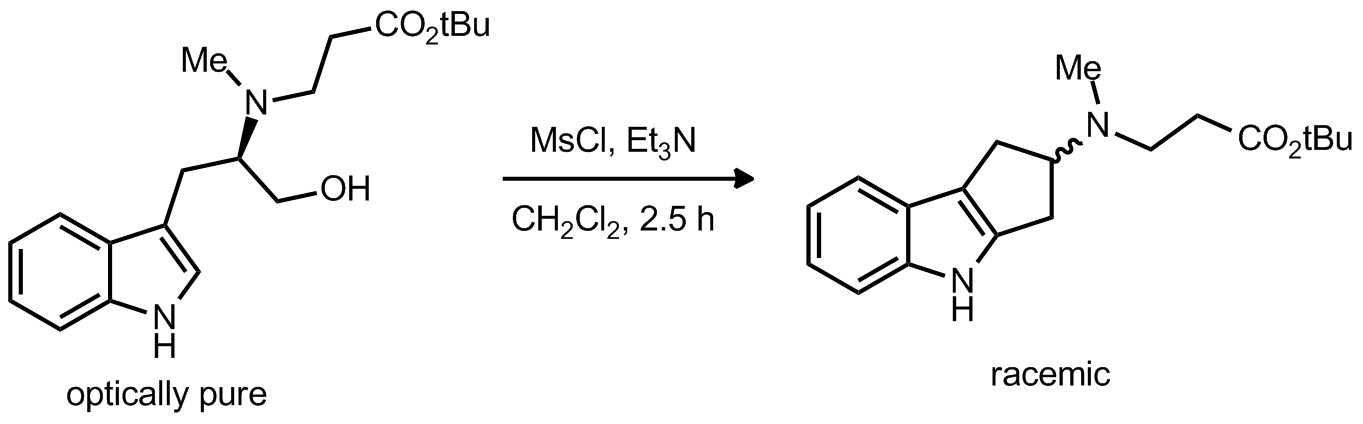
|
| Problem 2. Provide a mechanism for the following transformation Contributor: AJ Boydston Source: Org. Lett. J. Org. Chem. 1989, 54, 4298. Keywords: enamine, 1,4-addition, retro manich |
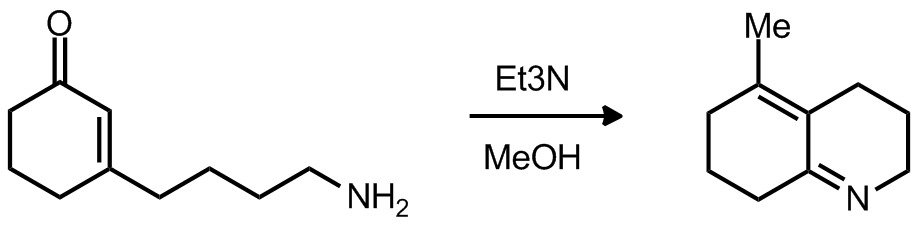
|
| Problem 1. Provide a mechanism for the following transformation Contributor: AJ Boydston Source: Synlett 1996, 521. Keywords: Horner�Wadsworth�Emmons, diazo |
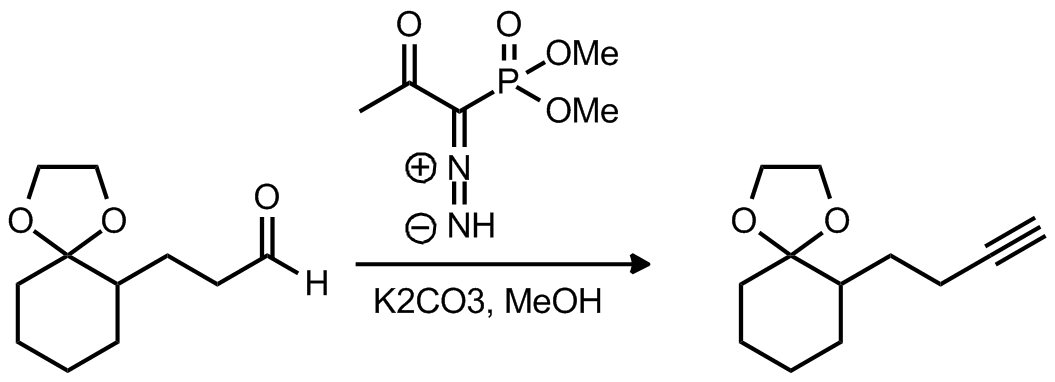
|
