Firstly, let us assume temperature doesn't change along the reactor, i.e., isothermal (it's not true, though). From the following table, we can see the reaction temperature affects the results tremendously.
| Temperature (K) | 473 | 673 | 873 | 1073 | 1273 | 1473 | 1673 | 1873 |
| Conversion | 0.08 | 0.72 | 1.0 | 1.0 | 0.99 | 0.92 | 0.84 | 0.78 |
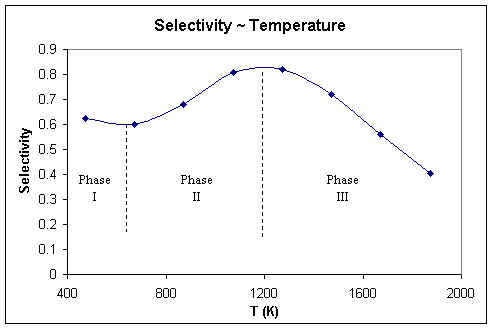
| Selectivity | 0.62 | 0.60 | 0.68 | 0.81 | 0.82 | 0.72 | 0.56 | 0.40 |
| Yield | 0.005 | 0.02 | 0.26 | 0.63 | 0.74 | 0.63 | 0.46 | 0.31 |
(Hey, what's the definition of conversion, selectivity and yield?)
When the temperature increases, the conversion increases first, up to 1(completely consumption), then falls down, which means the reactions speed up and then slow down. This is understandable if we see the reaction rates expression, because temperature term appears in both the denominator and numerator.
The change of selectivity is kind of interesting. Basically, the figure can be divided into 3 phases.

The decrease of selectivity in phase I is due to more importance of the reaction 2, but when temperature becomes higher (phase II), the selectivity increases. The reason for this is the reaction 1 is less exothermic than the reaction 2, so higher temperature is favorable to the reaction 1. But when temperature is too high (phase III), the reaction 3 will dominate, which cause the selectivity fall down again.
The effect on yield of NO is combination of conversion and selectivity. We can see, to obtain most products, the temperature cannot be too low or too high. The maximum yield happens at about 1273 K (1000 C).
The above discuss is based on isothermal condition, but the temperature does change in reactor, because reaction 1 and 2 are strongly exothermic. Want to see how it changes?