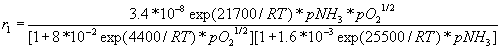
Reaction 1: NH3 + 5/4O2 ® NO + 3/2H2O + 226 kJ
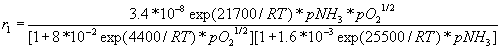
Reaction 2: NH3 + 3/2NO ® 5/4N2 + 3/2H2O + 454 kJ

Reaction 3: NH3 ® 1/2N2 + 3/2H2 – 47 kJ
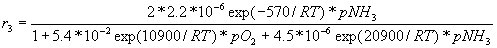
where
r = reaction rate, "mol/(sec.cm2)"
R = gas constant, = 1.987 cal/(mol.K)
T = temperature, "K"
p = pressure, "torr"