We identified ACE2 as the SARS-CoV-2 receptor and determined the viral spike glycoprotein cryoEM structure

Structural Biology of Infectious Diseases

We identified ACE2 as the SARS-CoV-2 receptor and determined the viral spike glycoprotein cryoEM structure
SARS-CoV and MERS-CoV human neutralizing antibodies revealed an unprecedented mechanism of receptor-functional mimicry.
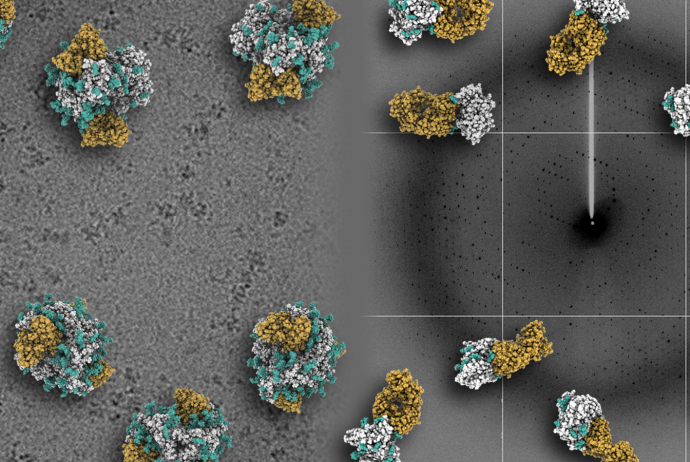
We showed how germline precursors of VRC01-class broadly neutralizing antibodies could bind to HIV envelope by tailoring the protein and glycan moieties.
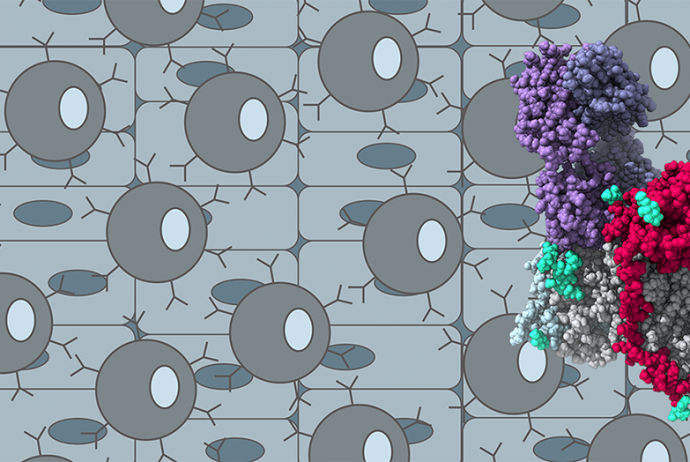
We determined the first structure of a human antibody in complex with the Epstein-Barr virus glycoproteins revealing an unprecedented mechanism of neutralization of dual-tropic infection.

Our work provides a structural framework to understand how enterotropic coronaviruses evolved to fine-tune fusion activation in the protease-rich environment of the small intestine.
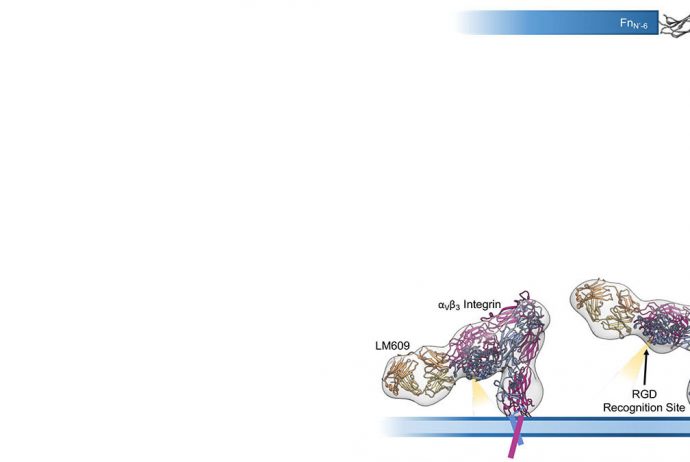
We characterized the molecular mechanism of human αVβ3 integrin inhibition by the candidate therapeutic antibody LM609.
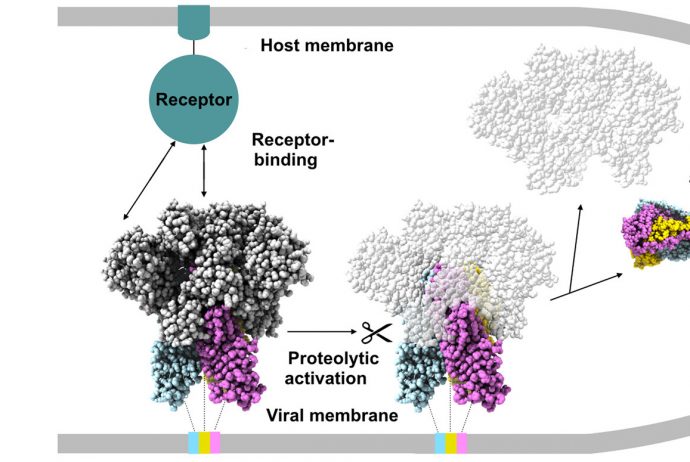
First cryoEM structure of a viral fusion protein in the postfusion conformation providing new insights about coronavirus infection.
We identified and characterized type VI secretion system effectors promoting Francisella growth in macrophages.
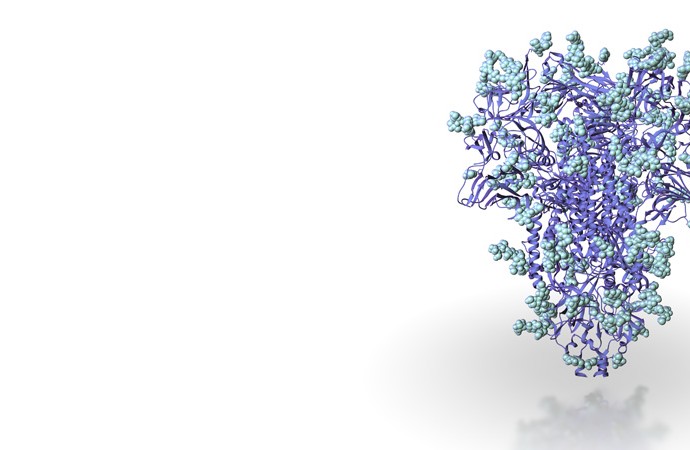
Our atomic resolution structure of a human coronavirus spike protein highlights strategies used by these viruses to evade the immune system.