Broadly speaking, we use the tools of biophysics and pharmacology to better understand how protein dynamics underlie biologically and clinically important phenomena such as pathological aggregation and functional heterogeneity. Here are a few of our most exciting active projects:
Capturing the Conformations of Tau. Tau is an intrinsically disordered protein (IDP) — instead of folding into a single, well-defined structure, tau rapidly samples a set of highly dissimilar structures. Tau is implicated in a range of neurodegenerative disorders collectively called tauopathies, which are characterized by the conversion of tau from its highly disordered monomeric form to dense intracellular aggregates (sometimes called 'tangles'). Perhaps the most well-known tauopathies are Alzheimer's disease and chronic traumatic encephalopathy (CTE; a consequence of repeated traumatic brain injuries, such as concussions).
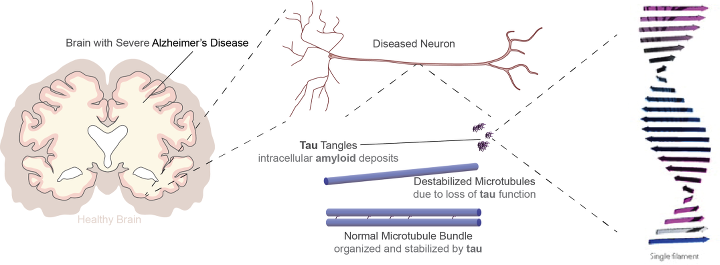
 Model of a drug-like small molecule bound to a short segment of tau
Unfortunately, tau is an extremely challenging drug target - its conformational plasticity and highly dynamic nature make conventional structure-aided drug design impossible.
Therefore, we are using single-molecule fluorescence and a battery of computational techniques to model the set of structures sampled by tau under different conditions, with the goal of defining locally structured parts of the protein at a high enough resolution to enable drug design and computational screening.
This work could lead to entirely new classes of therapeutics and diagnostics for Alzheimer's disease, CTE and other neurodegenerative disorders.
Model of a drug-like small molecule bound to a short segment of tau
Unfortunately, tau is an extremely challenging drug target - its conformational plasticity and highly dynamic nature make conventional structure-aided drug design impossible.
Therefore, we are using single-molecule fluorescence and a battery of computational techniques to model the set of structures sampled by tau under different conditions, with the goal of defining locally structured parts of the protein at a high enough resolution to enable drug design and computational screening.
This work could lead to entirely new classes of therapeutics and diagnostics for Alzheimer's disease, CTE and other neurodegenerative disorders.
Tau-Chaperone Interactions. Heat shock proteins and other chaperones are a crucial part of the body's defenses against protein misfolding and aggregation. In particular, intracellular chaperones such as Hsc70 play important roles in preventing tau aggregation. In collaboration with the Klevit lab (UW Biochemistry), we are using a combination of single-fluorescence and NMR experiments to better understand how chaperones and tau interact, and explore the mechanisms by which chaperones preserve tau in a non-pathological state. Modulating the activity of suitable chaperones could serve as another strategy for the management and treatment of tauopathies.
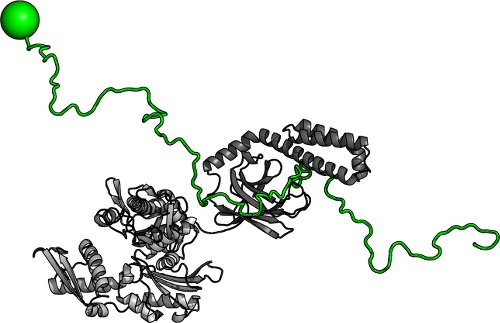
Molecular chaperones such as Hsc70 (grey) are capable of inhibiting tau (green) aggregation
Mechanisms of Amyloid Inhibition. Tau is one of several proteins that form a type of aggregate called amyloid. Amyloid deposition is a hallmark of many severe degenerative diseases (in addition to the taupathies mentioned above) such as Parkinson's disease, Pick's disease, type II diabetes, and Huntington's disease. The formation of amyloid is a fairly complicated process, typically proceeding through a set of oligomeric intermediates that are much more toxic than the final fibrillar deposits. We are developing general methods to identify which of the several phases of amyloid formation are affected by a given ligand (such as a drug candidate or a natural product). Our initial model systems are tau and SEVI (an amyloid-forming peptide with an intriguing link to HIV infectivity).
Functional Heterogeneity of Detoxification Enzymes. The more we learn about enzymes, the more complex their behavior appears. For instance, single-molecule experiments have shown that many enzymes are heterogeneous in substrate binding and catalysis — that is, two chemically identical protein molecules may display very different binding affinities and catalytic activities towards a given substrate. This ubiquitous behavior may be linked to allosterism, a phenomenon wherein ligand binding can modulate the affinity and activity of an enzyme towards its substrate. Many enzymes are also heterogeneous with respect to their choice of substrate — rather than being perfectly specific for a single substrate, they can accomodate a range of different molecules, sometimes dramatically different from each other.
We are interested in how the conformational dynamics and plasticity of enzymes underlie these fascinating behaviors. As with our studies of IDPs, single-molecule fluorescence and molecular simulations are well-suited to address these questions. We use glutathione-S-transferases, a family of detoxification enzymes, as a model system — in additional to generating insight into fundamental biological phenomena, our studies on GSTs will inform our understanding of drug metabolism and the body's response to oxidative stress.