First, we describe the three chemical reactions that occur in
this microreactor.
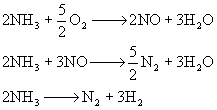
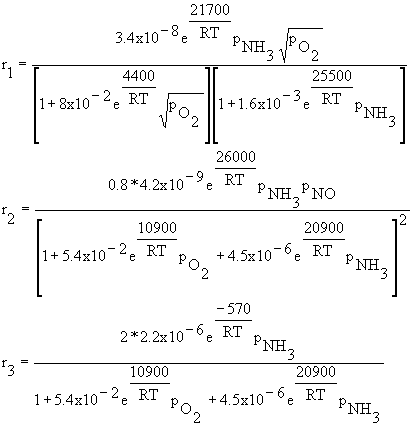
There are six molecules, each of which has a specific concentration profile
based on reaction rates.
The initial model will describe the flow rates of all species down the length
of the channel as the reactions proceed, given that the species present
initially are ammonia and oxygen. A differential equation is used to describe
the behavior of each species. This results in six coupled differential equations,
which must be solved simultaneously.
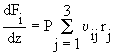
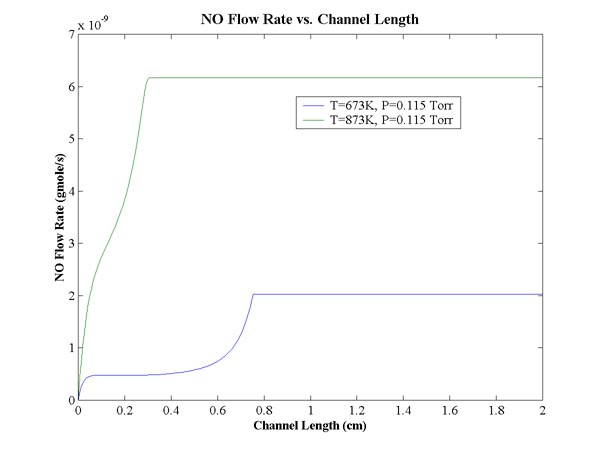
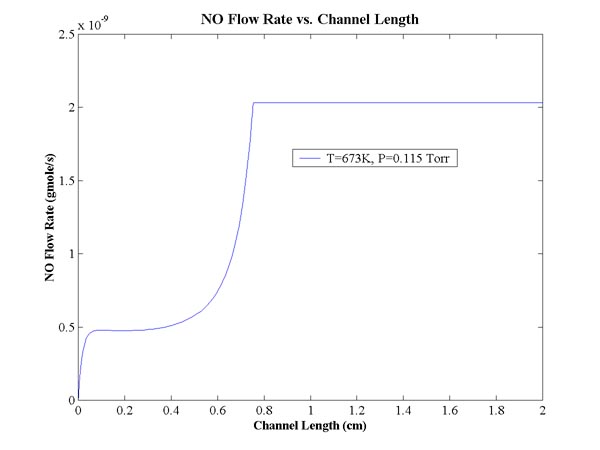
A program was written in Matlab to solve the system of equations numerically.
The result of the nitric oxide flow rate is shown with constant pressure and
temperature below.
We can change the temperature of the reactor to 873K and compare the results.