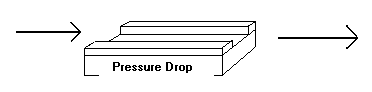
For our second model we removed the assumption of constant pressure.

We introduced a constant pressure drop that resulted in the pressure at the exit of the reactor being one half that of the inlet pressure:
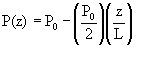
This resulted in a seventh ODE being added to our original concentration equations:
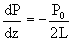
These seven ODEs were then solved in Matlab using ODE45. The optimal conditions for the reaction were determined to be constant pressure. Thus, the consequences of adding pressure drop were that as the pressure decreased, the concentration of NO decreased. Click below to see how pressure drop affects NO concentration.
| Return to Temperature/Concentration | Go on to Heat Generation |