Information + health
I was born in 1980 at a hospital in rural Oregon. As is still current practice, the staff overseeing my birth peeked at my anatomy, and checked “male” on my birth certificate. For the next 38 years, this one bit of binary health data was the basis for a profound number of experiences in my life. It was entered on forms registering me for public school. My pediatricians used this bit, without ever actually inspecting my body, to shape their preventative health care decisions about my weight, mental health, and risks of eating disorders. And when I turned 18, the government used this bit in its decision to send me U.S. military draft card. This single bit of binary data, far more than my body, shaped the course of my childhood and adolescence, both in health care and beyond. And, of course, I wasn’t a boy, or a man; as a transgender person, that bit was wrong, and it ended up defining more than how people treated me: it ended up defining how I saw myself in devastating ways.
Racing forward to my late thirties, I finally fixed that bit, correcting my birth certificate, health records, my government identification. And because in health care, it more often data that drives decisions, the health care system now treats me as the woman that I am. There’s just one problem: because health information technology is still binary about gender, it leaves no room for the sexual and gender diversity. I have a prostate gland, but I no longer get reminders about prostate exams, and my doctors no longer ask. I do not have a cervix, but I regularly get urgent reminders about overdue pap smears. Physicians unfamiliar with my trans body ask my why I don’t have a gynecologist and I get confused stares in the lobbies of urologists. And when I ask providers about gender-influenced health concerns about heart disease, kidney stones, osteoporosis, and strokes, they either shrug, and say, “There’s no science on your trans body” or they just assume the binary science on gender difference applies, treating me like every other woman.
These experiences, far from being about transgender health alone, reveal the complex interactions between health and information. Data about health is often used as a proxy for our anatomy and physiology, and when that data is biased, it biases care for our health and wellness. Data about our bodies, whether it’s carefully measured or simply assumed by someone looking at our bodies, is used to inform decisions outside health care, in education, work, and government. And science about health, which is our primary guide in shaping health care, is only as good as the data we collect. In the rest of this chapter, we will survey these complexities, mapping their interactions across different scales of our bodies, our selves, our health care systems, and our communities.
DNA as Information
In one sense, all life on Earth is partially influenced by information stored in DNA. First discovered in 1869 by Swiss chemist Friedrich Miescher, it was first called “nuclein”, as it was found in the nuclei of human white blood cells 14 14 Leslie A. Pray (2008). Discovery of DNA structure and function: Watson and Crick. Nature Education.
Wynand P. Roos, and Bernd Kaina (2006). DNA damage-induced cell death by apoptosis. Trends in molecular medicine.
The discovery of DNA has had profound effects on the science of life. For example, biology shifted a discipline of subjective description and classification to one of data analysis. This practice, now called bioinformatics , entails using machines to sequence DNA, algorithms and statistics to analyze DNA sequences, and the broader science of genomics to understand the particular sequences of DNA that contribute to disease 1 1 Andreas D. Baxevanis, Gary D. Bader, David S. Wishart (2020). Bioinformatics. John Wiley & Sons.
Duojiao Wu, Catherine M. Rice, and Xiangdong Wang (2012). Cancer bioinformatics: A new approach to systems clinical medicine. BMC Bioinformatics.
Understanding DNA as data also led to the record fast production of the SARS-CoV-2 vaccines in 2020. The virus, first detected by Chinese scientists, was known to have a distinctive “spike”; they quickly sequenced the 29,903 bases of its RNA and shared it globally on January 10th, 2020, just one month after the first identified case. Hundreds of scientists, using methods of bioinformatics, quickly determined that its RNA sequences were very similar to a previously known virus, SARS, another coronavirus. This led to the name SARS-CoV-2. German virologist Christian Drosten, who had long studied coronaviruses , developed the first test for this virus a few weeks later, by detecting particular bases in the virus’s genome. And then, using technology from the 1990’s to create mRNA vaccines that would inject instructions for human cells to producing the innocuous coronavirus spike, Pfizer and BioNTech partnered to construct the first vaccine in just a few weeks. The vaccine, essentially a sequence of RNA wrapped in a fatty acid, teaches our cells how to build the spike, which triggers an immune response that teaches our body to recognize and attack the full virus. The next 6 months involved clinical trials to verify the efficacy of the vaccines. None of this would have been possible, especially so fast, without understanding that DNA and RNA are essentially data. (Nor would it have been possible without decades of public investment in basic research by governments around the world, especially the United States and China.)
Health research as information
How do we know that a vaccine works? Or that any health care practice works? This knowledge, and the processes by which we produce it, are the foundation of modern medical practice. But for most of human history, we lacked this foundation 2 2 Arun Bhatt (2010). Evolution of clinical research: a history before and beyond James Lind. Perspectives in Clinical Research.
These superstitious practices began to shift in the 18th century. In 1747, physician James Lind was trying to address scurvy amongst sailors; amidst the backdrop of the broader scientific revolution of the 18th century, he decided to compare 12 similar patients, dividing them into each given different groups, and giving each different treatments. The ones who were given oranges and lemons were the only ones that recovered quickly led to the first evidence of scurvy as a vitamin-C deficiency. This was one of the first examples of a controlled experiment , or what health sciences usually calls a clinical trial .
The practice of clinical trials advanced from there. In 1811, the first documented use of placebos occurred, using deception to give a treatment that is known to be ineffective, but present it as the treatment being tested. In 1943, the first double-blind trial occurred, ensuring that both the patients and the providers giving treatments, were not told whether the treatment was a placebo. In 1946, the first randomized controlled trial occurred, in which the assignment of patients to the placebo or treatment group was random rather than self-selected or provider selected. All of these improvements, and the countless others that have followed since, have greatly increased the confidence and certainty of the methods by which scientists deem interventions to be effective. And this entire history of producing knowledge was brought to bear in evaluating the SARS-CoV-2 vaccines, to the point where universities and companies have well-established procedures and infrastructure, government units like the U.S. Food and Drug Administration have well-established regulations for evaluating clinical trial results, and medical journals, read widely by medical providers, have well-established norms for objectively reviewing and publishing the results of trials.
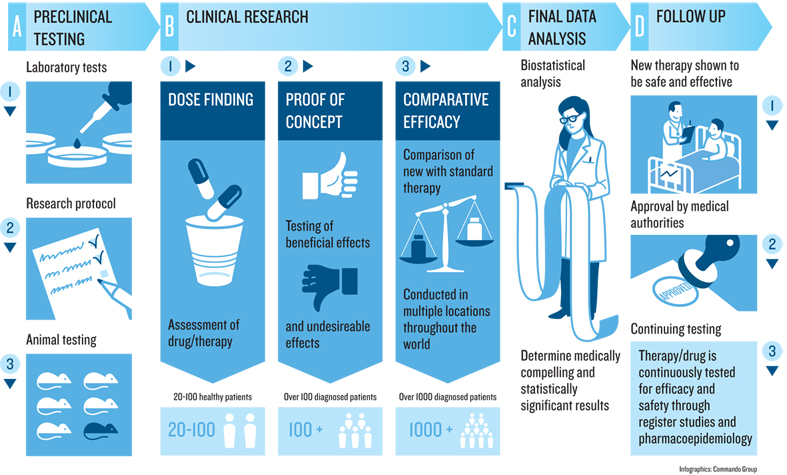
While clinical trials are the highest standard in producing knowledge about health interventions, there are countless other research methods used to build knowledge about health and medicine. Descriptive studies can identify new phenomena and theories about health. Correlational studies,—like the kind often reported in journalism indicating that eating some food increases or decreases the risk of some disease—can help generate hypotheses to be later tested in randomized controlled experiments. And qualitative methods, such as interviews, field observations, and ethnographies, can help identify social, cultural, and organizational factors that shape the efficacy of interventions. For example, it doesn’t matter if a drug is effective if someone doesn’t take it correctly, forgets to take it because of a complex dosing schedule, or did not keep the instructions on how to take it because they are homeless. Qualitative methods shape our understanding of why a medication does or doesn’t work, as the reasons are often sociocultural, not physiological.
Regardless of method, the same values, biases, and systems of oppression that affect all data, information, and knowledge, affect health knowledge, resulting in science that supports and highlights the experiences of dominant groups, while often disregarding or erasing the experiences of marginalized groups, or even doing direct harm. For example, a study conducted between 1932 and 1972 of syphilis recruited Black men, telling them that they were receiving free health care from the federal government. The reality, however, was that the government study was observing the natural course of syphilis, and administered no care when it was detected, did not notify the men that they had detected it, and even offered placebos that were known to be ineffective. As a result, hundreds of men died of syphilis, forty partners contracted the disease, and 19 children were born with congenital syphilis. The focus on Black men was explicitly racist 3 3 Allan M. Brandt (1978). Racism and research: the case of the Tuskegee Syphilis Study. Hastings Center Report.
Vicki S. Freimuth, Sandra Crouse Quinn, Stephen B. Thomas, Galen Cole, Eric Zook, and Ted Duncan (2001). African Americans’ views on research and the Tuskegee Syphilis Study. Social Science & Medicine.
Information in health care
From both a patient, modern health care is rife with information problems. When we experience symptoms in our health, we rely on information from friends, family, and increasingly the internet to even decide whether to utilize health care services 17 17 Kendra L. Schwartz, Thomas Roe, Justin Northrup, James Meza, Raouf Seifeldin, and Anne Victoria Neale (2006). Family medicine patients’ use of the Internet for health information: a MetroNet study. The Journal of the American Board of Family Medicine.
Michael Marmot, Richard Wilkinson (2005). Social determinants of health. OUP Oxford.
Of course, the information-driven processes above are rarely so seamless. Patients and clinicians often lack a shared understanding of a patient’s goals 13 13 Ari H. Pollack, Sonali R. Mishra, Calvin Apodaca, Maher Khelifi, Shefali Haldar, and Wanda Pratt (2020). Different roles with different goals: Designing to support shared situational awareness between patients and clinicians in the hospital. Journal of the American Medical Informatics Association.
Shefali Haldar, Sonali R. Mishra, Maher Khelifi, Ari H. Pollack, and Wanda Pratt (2019). Beyond the Patient Portal: Supporting Needs of Hospitalized Patients. ACM Conference on Human Factors in Computing Systems.
Lisa M. Vizer, Jordan Eschler, Bon Mi Koo, James Ralston, Wanda Pratt, and Sean Munson (2019). “It’s Not Just Technology, It’s People”: Constructing a Conceptual Model of Shared Health Informatics for Tracking in Chronic Illness Management. Journal of Medical Internet Research.
Daniel A. Epstein, Monica Caraway, Chuck Johnston, An Ping, James Fogarty, and Sean A. Munson (2016). Beyond abandonment to next steps: understanding and designing for life after personal informatics tool use. ACM Conference on Human Factors in Computing Systems.
Helena M. Mentis, Anita Komlodi, Katrina Schrader, Michael Phipps, Ann Gruber-Baldini, Karen Yarbrough, and Lisa Shulman (2017). Crafting a View of Self-Tracking Data in the Clinical Visit. ACM Conference on Human Factors in Computing Systems.
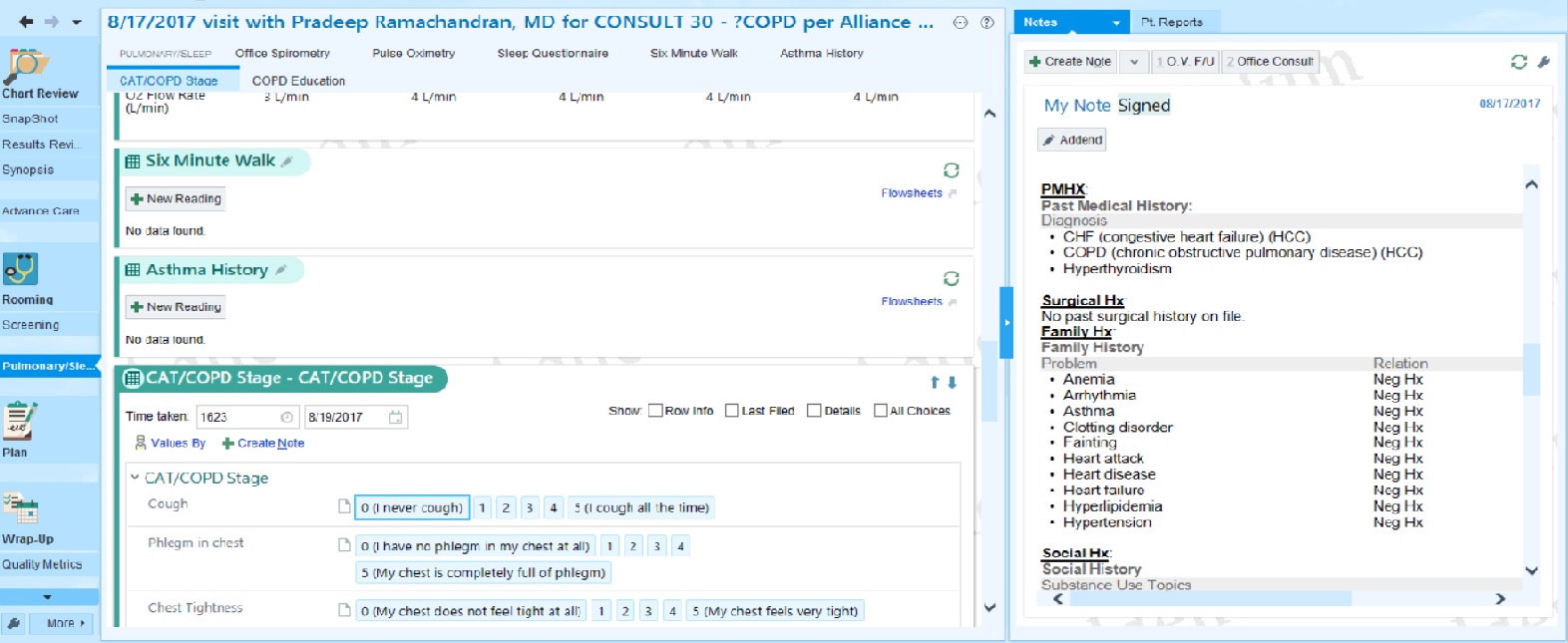
The provider side of health care from inside these information systems is similarly complex. Providers and hospitals often have few ways to overcome the poor design and usability of electronic medical records other than simply hiring more staff to workaround these problems 16 16 Gordon D. Schiff, and Laura Zucker (2016). Medical scribes: salvation for primary care or workaround for poor EMR usability?. Journal of General Internal Medicine.
Helena M. Mentis, Madhu Reddy, and Mary Beth Rosson (2010). Invisible emotion: information and interaction in an emergency room. ACM Conference on Computer Supported Cooperative Work and Social Computing.
William Hersh (2004). Health care information technology: progress and barriers. Journal of the American Medical Association.
Atop all of these challenges with individual patients are population health challenges 9 9 David Kindig and Greg Stoddart (2011). What is population health?. American Journal of Public Health.

At the foundation of all of these intersections between information and health are issues of diversity, equity, and inclusion. Biology is inherently diverse, human experiences are inevitably diverse, and yet so much of our understanding of DNA, effective medicine, and population health stems from studies that often systematically exclude significant parts of that diversity, or erase it through aggregate statistics. These practices of supporting health through a lens of dominant groups results in systemic inequality in who is treated, who is treated effectively, and therefore who lives long, healthy lives. Challenges in information and health, therefore, are fundamentally health equity challenges 4 4 Paula Braveman (2006). Health disparities and health equity: concepts and measurement. Annual Review of Public Health.
Podcasts
For more about the intersection of health and information, consider these podcasts:
- The Ashes on the Lawn, Radiolab . Describes the history of HIV/AIDS advocacy, and the response of the U.S. federal government to this advocacy.
- The Great Vaccinator, Radiolab . Describes the contributions of Maurice Hilleman, inventor of more than 40 vaccines, many routinely given to children.
- The Science Behind The Historic mRNA Vaccine, Short Wave . Describes the science behind the mRNA technology used in the Pfizer-BioNTech COVID-19 vaccine.
- Down and Dirty with Covid Genes, In Machines We Trust, MIT Technology Review . Describes the many ways that DNA scrubbing and machine learning is being used to detect and treat disease.
- The Rise of Therapy Apps, What Next TBD, Slate . Discusses a new genre of therapy apps intended to scale access to mental health, as well as their limitations, and the risks of lowering the bar on care.
- How AI is giving a woman back her voice, In Machines We Trust . Discusses applications of voice recognition in health care.
- What Does It Mean to Give Away Our DNA?, The Experiment . Discusses the tensions between genetic testing and Indigenous communities.
- The Downfall of One of the World’s Biggest Brains, What Next TBD . Discusses IBM Watson’s failulre to transform health care.
References
-
Andreas D. Baxevanis, Gary D. Bader, David S. Wishart (2020). Bioinformatics. John Wiley & Sons.
-
Arun Bhatt (2010). Evolution of clinical research: a history before and beyond James Lind. Perspectives in Clinical Research.
-
Allan M. Brandt (1978). Racism and research: the case of the Tuskegee Syphilis Study. Hastings Center Report.
-
Paula Braveman (2006). Health disparities and health equity: concepts and measurement. Annual Review of Public Health.
-
Daniel A. Epstein, Monica Caraway, Chuck Johnston, An Ping, James Fogarty, and Sean A. Munson (2016). Beyond abandonment to next steps: understanding and designing for life after personal informatics tool use. ACM Conference on Human Factors in Computing Systems.
-
Vicki S. Freimuth, Sandra Crouse Quinn, Stephen B. Thomas, Galen Cole, Eric Zook, and Ted Duncan (2001). African Americans’ views on research and the Tuskegee Syphilis Study. Social Science & Medicine.
-
Shefali Haldar, Sonali R. Mishra, Maher Khelifi, Ari H. Pollack, and Wanda Pratt (2019). Beyond the Patient Portal: Supporting Needs of Hospitalized Patients. ACM Conference on Human Factors in Computing Systems.
-
William Hersh (2004). Health care information technology: progress and barriers. Journal of the American Medical Association.
-
David Kindig and Greg Stoddart (2011). What is population health?. American Journal of Public Health.
-
Michael Marmot, Richard Wilkinson (2005). Social determinants of health. OUP Oxford.
-
Helena M. Mentis, Madhu Reddy, and Mary Beth Rosson (2010). Invisible emotion: information and interaction in an emergency room. ACM Conference on Computer Supported Cooperative Work and Social Computing.
-
Helena M. Mentis, Anita Komlodi, Katrina Schrader, Michael Phipps, Ann Gruber-Baldini, Karen Yarbrough, and Lisa Shulman (2017). Crafting a View of Self-Tracking Data in the Clinical Visit. ACM Conference on Human Factors in Computing Systems.
-
Ari H. Pollack, Sonali R. Mishra, Calvin Apodaca, Maher Khelifi, Shefali Haldar, and Wanda Pratt (2020). Different roles with different goals: Designing to support shared situational awareness between patients and clinicians in the hospital. Journal of the American Medical Informatics Association.
-
Leslie A. Pray (2008). Discovery of DNA structure and function: Watson and Crick. Nature Education.
-
Wynand P. Roos, and Bernd Kaina (2006). DNA damage-induced cell death by apoptosis. Trends in molecular medicine.
-
Gordon D. Schiff, and Laura Zucker (2016). Medical scribes: salvation for primary care or workaround for poor EMR usability?. Journal of General Internal Medicine.
-
Kendra L. Schwartz, Thomas Roe, Justin Northrup, James Meza, Raouf Seifeldin, and Anne Victoria Neale (2006). Family medicine patients’ use of the Internet for health information: a MetroNet study. The Journal of the American Board of Family Medicine.
-
Lisa M. Vizer, Jordan Eschler, Bon Mi Koo, James Ralston, Wanda Pratt, and Sean Munson (2019). “It’s Not Just Technology, It’s People”: Constructing a Conceptual Model of Shared Health Informatics for Tracking in Chronic Illness Management. Journal of Medical Internet Research.
-
Duojiao Wu, Catherine M. Rice, and Xiangdong Wang (2012). Cancer bioinformatics: A new approach to systems clinical medicine. BMC Bioinformatics.