The Effect of Pressure Drop
Now let’s see how much pressure drop affects the trends in conversion, selectivity and yield.
| Tin(K) | 473 | 673 | 873 | 1073 | 1273 | 1473 | 1673 | 1873 |
| Texit (K) | 1561 | 1757 | 1837 | 1901 | 1955 | 2005 | 2058 | 2121 |
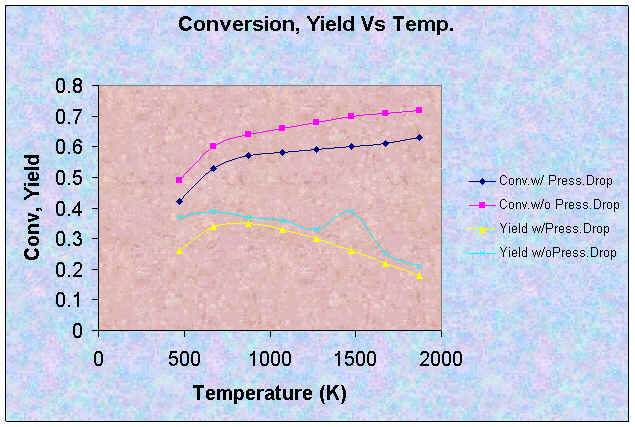
| Conversion | 0.42 | 0.53 | 0.57 | 0.58 | 0.59 | 0.60 | 0.61 | 0.63 |
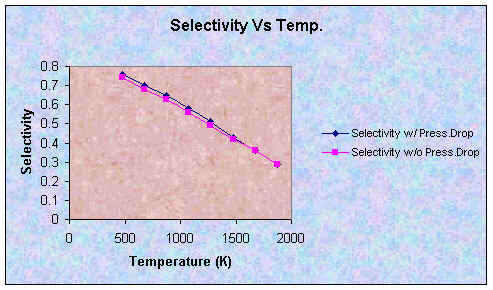
| Selectivity | 0.76 | 0.70 | 0.65 | 0.58 | 0.51 | 0.43 | 0.36 | 0.29 |
| Yield of NO | 0.26 | 0.34 | 0.35 | 0.33 | 0.30 | 0.26 | 0.22 | 0.18 |
(Hey, what's the definition of conversion, selectivity and yield?)
From the results, adding a pressure drop effect into our calculations does not change the trend at all. However, the effect of pressure drop decreases the values for conversion and yield. On the other hand, it increases the values for selectivity. This is reasonable because the pressure drop causes the temperature not to increase as rapidly, as proven by lower exit temperatures. Thus, reactions can not go as fast as if there is no pressure drop which results in lower conversions and yield. This effect also causes Reaction 3 to be less important, which increases selectivity.
Moreover, the pressure drop effect lowers the maximum yield to 0.35, which is about 10% lower than the original value. The maximum yield occurs at T = 873 K.
The effects can be easily seen in the plots below.