


Reaction Mechanism
Polyethylene is an addition polymer that is created by the polymerization of ethylene monomer units as its name implies. Ethylene can be polymerized by a radical mechanism under very high pressures and temperatures with the addition of an organic peroxide radical initiator. This reaction is thought to proceed as follows where the arrows indicate single electron flow:Step 1. Initiation. Dissociation of the peroxide's oxygen bonds leads to a temporary intermediate, which then is quickly converted into carbon dioxide and 2 radicals:
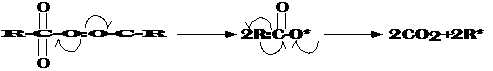
* indicates an unpaired electron and R represents a carbon group.
Step 2. Initiation. These radicals can then act as electrophiles and attack the double bond of ethylene forming a stabler intermediate:
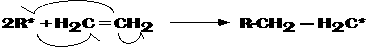
Step 3. Propagation. The newly formed radical can add on to itself by attacking another ethylene molecule:

Polyethylene
units can grow from 4-5 monomer units to very large molecules that have
molecular weights as large as 1 million. The number of ethylene monomer
units and hence the molecular weight in the polyethylene molecule can be
contolled by the reaction temperature, the type of catalyst employed, and
the radical concentration. Step 4. Termination. Finally the
reaction is terminated when two radicals react with each other to produce
a product with paired electrons:
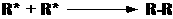
The reaction steps are briefly summarized below:
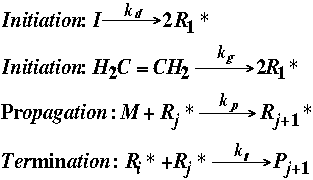
If we assume the above are elementary reactions, the rate laws can be written as follows:
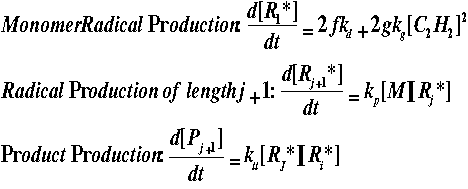 where f, g represent efficiency factors and k represents rate of reaction
constants.
where f, g represent efficiency factors and k represents rate of reaction
constants.
Algebraic manipulation of the above equations results in the final flow design equations:
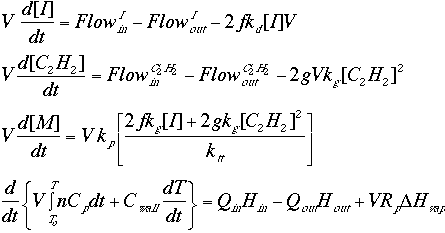
where V is the volume of the reactor.
It should be noted that these equations pertain to the high pressure polymerization process, as rate law literature on the metallic catalyst process is extremely rare. However, if experimental data on the rate laws for the metallic catalyst process is available, these flow design equations can be modified to encompass these new rate laws.
Click mathematical models to return.
Click reactor to return to the fluidized reactor home page.
