

Research Areas
The focus of the Hinds’ group research program is to fabricate functional materials at the nanometer scale. Functionality is found through information processing, chemical sensing, and active chemical separation. The key challenges are developing architectures that couple activity on the nm-scale to the larger macroscopic world.
With these goals, we have two focused areas of research of Carbon Nanotube based Membranes and Molecular Scale Electrodes.
Carbon Nanotube based Membranes
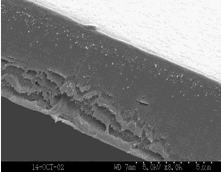
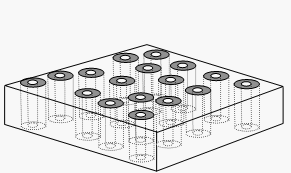
'Dramatic Transport Properties of Carbon Nanotube Membranes for a robust protein channel mimetic platform’ B.J. Hinds* Curr. Opin. in Solid. State & Mater. Sci. 2012 16(1) 1-9. (pdf file)
A major research effort for functional nano-materials in my group is based on the invention of an aligned multiwalled carbon nanotube membranes using simple polymer processing [32]. Essentially, an already aligned array of CNTs is impregnated with polymer (without disrupting the alignment), removed from substrate, and the surfaces are plasma oxidized to open the previously closed CNT cores. The figure above shows a cross-sectional SEM of the membrane and a schematic of the idealized structure. There are three key attributes unique to CNT membranes:
1) Atomically flat hydrophobic graphitic core
2) Functional chemistry by necessity is at the cut entrances to the CNT cores
3) CNTs are conductive allowing for electrochemical transformation and application of electric field
This CNT platform can closely mimics biological channels that have receptor chemistry, extremely fast mass transport/pumping through the core and signal chemistry valves. In particular, a structure that allows ‘gatekeeping’ can solve one of the long-standing trade-offs in separation science: high selectivity generally results in low flux rates due to increased interactions along the pore length. With a highly selective gatekeeper, selective interactions will occur only at pore entrances allowing for rapid transport through the pore. In principle, by changing the chemistry at the entrance to CNT core, we can change the membrane’s application. Funded projects in the Hinds’ group include water desalination, forward osmotic power generation, chemical separations (including chiral), catalysis, energy storage, and programmed drug delivery.
After the initial report of the membrane fabrication [32], the primary research goals have been to 1) understand basic transport mechanisms through the CNT cores and 2) demonstrate gatekeeper activity at the pore entrance. To demonstrate activity of the CNT membranes, functional chemistry tethered to the CNT core was systematically changed. The principle conclusions from these studies [37, 55, 58] are that gasses are enhanced over Knudsen diffusion by one order of magnitude due to specular reflection off of an atomically flat surface. For fluid flow, the enhancement is extreme with 4-5 orders of magnitude increase in flux due to near perfect slip boundary [39] which was independently confirmed by LLNL (Holt et al. Science 2006). Other notable studies show reversible biochemistry at pore entrances that can detect proteins or their release agent [36] and that a full ATP phosphlyation cycle can be performed on tethered polypeptide gating the flow through the CNT core [43]. Also we showed that we could independently functionalize each side of the membrane separately [35]. A significant research accomplishment was the demonstration of electrostatically actuated gatekeepers to open and close the pore entrance [44]. In that study we showed that we can force the electrochemical functionalization to occur only at the tip entrance by having a fast fluid flow of inert solvent through the core during electrochemical grafting. Thus, the basic building blocks for the intellectual grand challenge of mimicking natural protein channels exist in the CNT membrane system: independent gate-keeper/signal chemistry on each side of the channel and enhanced transport properties through the channel core.
An important intellectual puzzle immerged; by adding functional chemistry within the CNT to get chemical selectivity we destroy the ‘near perfect’ slip boundary condition and reduce the fluid flow enhancement by 2-4 orders of magnitude [55] …. thereby eliminating the key merit of the CNT system. Fortunately we know that protein channels can have high flow rates and selectivity due to an ability to pump/accelerate chemicals through the region of strong chemical interaction. To mimic this approach we have started with the phenomena of electroosmosis (EO) and electrophoresis (EP) to pump/accelerate neutrals/ions past a selective gatekeeper. The near ‘ideal’ slip boundary condition can support electroosmotic flow without surface scattering. Recent results have shown that the CNTs have very energy efficient EO with 1-3 orders of magnitude more energy efficiency [56] than comparable nanoporous membranes. This phenomenon is at the heart of our PNAS report about programmed pumping of nicotine through CNTs at therapeutically useful doses for addiction treatment [50]. The dramatic improvement in power efficiency allows a watch battery to pump a skin patch device for over 10 days. The key merit of such a device is that addiction treatment is a complex mixture of psychological counseling and chemical physiology. Such a device can be programmed, within prescription limits, through remote/online counseling bringing psychology and physiology together. This project was the subject of the NIH sponsored PECASE award. Electroosmotic flow (ions pumping neutral solvent/molecules) is dramatically enhanced by 3-4 orders of magnitude consistent with the pressure driven studies [59] and allows extremely power efficient pumping. The electrophoresis phenomenon was also seen to be efficient for biomolecule separations with the first demonstration of protein separations with CNT membranes [53]. By taking advantage of diazonium electrochemical grafting chemistry we have been able to uniformly deposit, down to a monolayer, Pt catalyst on CNT surfaces resulting in the highest reported mass activity on CNTs for MeOH fuel cells [52, 60]. In general, future research directions for the CNT membranes are summarized as following:
a) Chemical modification of CNT tips for chemical separations
b) Electrochemical grafting to improve functional density and controlled macromolecular synthesis at pore entrance
c) Electrostatic, electroosmotic and electrophoretic studies in CNT transport and incorporation into programmable transdermal drug delivery
d) Chiral catalysis and separation
e) Electrochemical transformation and separation (fuel cells and solar energy storage)
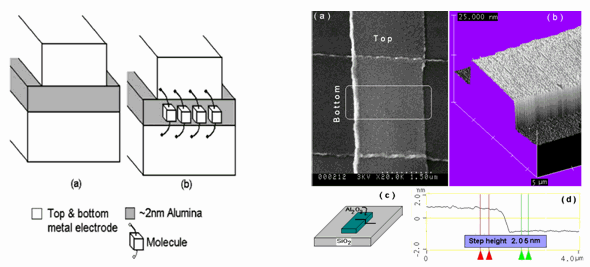
With the advent of scanning probe techniques such as Atomic Force Microscopy (AFM) and Scanning Tunneling Microscopy (STM), remarkable achievements in isolating and manipulating single molecules and atoms has been accomplished. However, by the nature of a scanning probe, only 1 molecule can be seen at a time that makes it impossible to scale up for computer devices. The tip of an AFM/STM can be used to draw nano-scale lines but this is a very slow process. The analogy would be comparing the pen (AFM/STM) to the printing press (photolithograpy). What is needed is a lithography technique with the resolution to isolate a single molecule AND large area parallel exposures. We are currently working on a technique to use photolithographically defined structures that can result in sub-nm scale resolution. This would allow us to make a circuit of isolated molecules.
The figure shown above schematically shows the novel approach for molecular electrodes [40]. The fundamental idea is to use film thickness of an insulator (on the exposed surface of a patterned multilayer edge) to set the critical electrode dimension to match that of the molecule’s length. The key experimental goals have been to: 1) demonstrate functioning molecular electrodes at the edge of multilayer patterns [40] 2) control location and diameter of nanowires for shadow lithography. A novel approach to control the CNT diameter is to use a film thickness of catalyst layer (in a multilayer structure) to set the diameter of CNTs growing laterally from the pattern edge [31, 33]. The geometry of line-of-site shadow evaporation with CNT masks was also confirmed directly in a TEM study [34]. Other nanowire material systems were also explored such as CuO [43] and ZnO (EMC presentation 2006).
With further processing steps it is possible to isolate a single molecule and study electron conduction through it. In particular, the self-assembly of coordination compounds would be most interesting especially considering the role of the spin of the electron. By using thin-film growth techniques, multilayers of magnetic films can control and detect the spin of electrons traveling through the isolated molecule. Applications include Giant Magneto Resistance, which is the basis of the read-head in the hard drive of the computer you are using to read this web page.
Important links to research centers that I am associated with
Chemical & Materials Engineering Dept. UKY
Advanced Science and Technology Commercialization Center (ASTeCC)
Center for Nanoscale Science and Engineering (CeNSE)
Center for Advanced Energy Research (CAER)
ElectroOptics Research Institute (Univ. of Louisville)
60. “Pt monolayer deposition onto carbon nanotube mattes with high electrochemical activity” Xin Su, Xin Zhan, Bruce J. Hinds* J. Mater. Chem. In press DOI:10.1039/C2JM15395E
59. “Electrophoretically Induced Aqueous Flow through sub-Nanometer Single Walled Carbon Nanotube Membranes” Ji Wu, Karen Gerstandt, Hongbo Zhang, Jie Liu, and Bruce. J. Hinds* Nature Nano 2012 7(2) 133-39.
58. ‘Dramatic Transport Properties of Carbon Nanotube Membranes for a robust protein channel mimetic platform’ B.J. Hinds* Curr. Opin. in Solid. State & Mater. Sci. 2012 16(1) 1-9.
57. ‘Highly Efficient Electro-osmotic Flow through Functionalized Carbon Nanotubes Membrane’ Ji Wu, Karen Gerstandt, Mainak Majunder, B.J. Hinds*, RCS Nanoscale 2011 3(8) 3321-28
56. ‘Simulation of steady state methanol flux through a model carbon nanotube catalyst support’ Jacob Goldsmith and B. J. Hinds* J. Phys. Chem. C 2011 115(39) 19158-64
55. ‘Mass Transport through Carbon Nanotube Membranes in three different regimes: ionic diffusion, gas, and liquid flow’ Mainak Majumder, Nitin Chopra, B.J. Hinds* ACS Nano 2011 5(5) 3867-3877
54. ‘Selective Lateral ZnO Nanowire Growth by Surface Diffusion on nm-Scale Patterned Alumina on Silicon’ Bing Hu, Nitin Chopra, Pawan Tyagi, B. J. Hinds* J. Mater. Res. 2011 26(17) 2224-31
53. ‘Electrophoretic Transport of Biomolecules through Carbon Nanotube Membranes’ Xinghua Sun, Xin Su, Ji Wu, B. J. Hinds* Langmuir 2011 27(6) 3150-56.
52. ‘Catalytic Activity of Ultrathin Pt Films on Aligned Carbon Nanotube Arrays‘ Xin Su, Ji Wu, B.J. Hinds* Carbon 2011 49(4) 1145-50.
51. ‘Nano-gap electrodes formed at the exposed edge of Au/SAM/Al2O3/Au tunnel structures grown by atomic layer deposition’ Bing Hu, Jingyuan Yao, B. J. Hinds* Appl. Phys. Letters 2010 97(20) 203111.
50. “ Programmable transdermal drug delivery of nicotine using carbon nanotube membranes” J. Wu, K.S. Paudel, C.L. Strasinger, D. Hamell, Audra L. Stinchcomb*, B. J. Hinds* Proc. Nat. Acad. Sci. 2010 107(26) 11698-11702. (Nature Materials Highlight)
49. “Mechanism of ultrathin tunnel barrier failure due to mechanical-stress-induced nanosized hillocks and voids” P. Tyagi and B.J. Hinds J. Vacuum Sci. & Techn. B 2010 28, 517-521.
48. “Towards mimicking natural protein channels with aligned carbon nanotube membranes for active drug delivery” Majumder, M; Stinchcomb, A; Hinds BJ* Life Sciences 2010 86, 563-68.
47 “Carbon Nanotube Membranes for use in the Transdermal Treatment of Nicotine Addiction and Opioid Withdrawal Symptoms.” C.L. Strasinger, N. N. Scheff, J. Wu, B. J. Hinds, Audra L. Stinchcomb*, Substance Abuse: Research and Treatment 2009:3 31–39
46. “Enhanced Electro-Static Modulation of Ionic Diffusion through Carbon Nanotube Membranes by Diazonium Grafting Chemistry” Majumder, M; Keis, K M.; Zhan, X; Hinds, B.J * J. Membr. Sci. 2008, 316, 89-96.
45. “A Blueprint for a Nanoscale Pump” Hinds, B* Nature Nanotechnology 2007, 2, 673-674
44. “Voltage Gated Carbon Nanotube Membranes” Majumder, M.; Zhan, X; Andrews, R; Hinds, B.J * Langmuir 2007; 23(16); 8624-8631.
43. “Kinetic Study of Copper Oxide Nanowire Growth from the Edges of Thin Film Multilayer Structures” Chopra, N; Hu, B.; Hinds B.J* J. Mater. Res.. in-press
42. “Carbon Nanotube Based Biomimetic Membranes: Mimicking Protein Channels Regulated by Phosphorylation” Nednoor, P; Gavalas, V.G; Chopra, N; Hinds, B.J.*, Bachas, L.G.* J. Mater. Chem.. 2007 17, 1755–1757
41. “Mechanical Stress Control for the Fabrication of Stable Molecular Electrodes at Patterned Edge of a Metal/Insulator/Metal Junction” Tyagi, P.; Li, D.; Holmes S.M.; Hinds, B.J.* IEEE Trans. Nanotech. revision in review.
40. “Molecular Electrodes at the exposed edge of metal-insulator-metal trilayer structures” Tyagi, P.; Li, D.; Holmes S.M.; Hinds, B.J.* J. Amer. Chem. Soc. 2007 129, 4929-4938.
39. “Nanoscale hydrodynamics: Enhanced flow in carbon nanotubes” Majumder, M.; Chopra, N.; Andrews, R; Hinds, B.J * Nature 2005, 438, 44.
38. “Raman Spectroscopic Investigation of Gas Interactions with an Aligned Multiwalled Carbon Nanotube Membrane” Matranga, C*; Bockrath, B; Andrews, R; Chopra, N, Hinds, BJ Langmuir 2006; 22, 1235-1240.
37. “Effect of Tip Functionalization on Transport through Vertically Oriented Carbon Nanotube Membranes” Majumder, M.; Chopra, N.; Hinds, B.J * J. Amer. Chem. Soc. 2005; 127, 9062-9070.
36. “Reversible Biochemical Switching of Ionic Transport through Aligned Carbon Nanotube Membranes” Nednoor, P.; Chopra, N.; Gavalas, V.; Bachas, L.G.*; Hinds, B.J.* Chem. Mater. 2005 17, 3595-3599.
35. “Bi-functional Carbon Nanotubes by Sidewall Protection” Chopra, N; Majumdar, M; Hinds, B.J.* Adv. Funct. Mater. 2005 15, 858-64.
34. “Effect of Incident Angle in Suspended Carbon Nanotube Shadow Lithography” Chopra, N; Xu, W; De Long, L.E.; Hinds, B.J* Nanotechnology 2005 16, 133-36.
33. “Catalytic size control of multiwalled carbon nanotube diameter in xylene chemical vapor deposition process” Chopra, N., Hinds, B.J.* Inorg. Chem. Acta 2004 357, 3920–3926.
32. “Aligned Multiwalled Carbon Nanotube Membrane” Hinds, B.J.*; Chopra, N.; Andrews, R.; Gavalas, V.; Bachas L. Science 2004 303 62-65.
31. “Control of Multiwalled Carbon Nanotube Diameter by Selective Growth on the Exposed Edge of a Thin Film Multilayer Structure” Chopra, N. Kichambare, P.D. Andrews, R. and Hinds, B.J.* Nanoletters 2002 2(10) 1177-1181.