|
Baneyx BioNano Laboratories.
|
|
..Molecular Chaperones
|
|
..The ClpB disaggregase
|
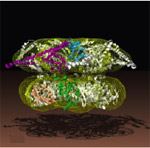
ClpB belong to the Hsp100 family of ring-forming heat shock proteins whose role is to either degrade unfolded/misfolded proteins, or to solubilize and reactivate aggregated proteins. ClpB, which functions independently of a protease component, is a hexameric ATPase involved in protein disaggregation. This activity is essential for cells to transiently survive extreme thermal stress. Current models hold that the DnaK-DnaJ-GrpE system plays a critical role in the early stages of the disaggregation reaction by facilitating ClpB-mediated extraction of single molecules from aggregates. The captured substrate is next threaded through the 1.6 nm central pore of ClpB where it undergoes net unfolding in a mechanical process that consumes ATP. As it exits the ClpB channel, the unfolded polypeptide may spontaneously refold or be transferred to the DnaK-DnaJ-GrpE and/or GroEL-GroES for chaperone-assisted refolding.
ClpB contains a highly mobile N-terminus domain that surrounds its pore and contributes to enhanced disaggregation activity against certain substrates. The N domain is however dispensable for aggregate solubilization (in fact E. coli synthesizes two versions of ClpB, one containing and one lacking an N domain, from the same mRNA). On the other hand, the ClpB M domain, a coiled coil structure that projects laterally from the top of the ring, appears to play an essential role in the disaggregation process by coupling DnaK-DnaJ-GrpE aggregate "feeding" and ClpB threading motor activity.
| Reconstructed structure of the ClpB hexamer. The N domain is not visible on the reconstruction. The M domain projects outwards from the "top" portion of the hexameric ring. The structure shown is from T. thermophilus and is discussed in more detail by Francis Tsai here. |

| | home | research | publications | people | covers | links | teaching | intranet |
Contact: François Baneyx, University of Washington, Department of Chemical Engineering, Box 351750, Seattle, WA Tel: 206-685-7659 Fax: 206-685-3451 E-mail: baneyx@u.washington.edu © 2008 François Baneyx - All Rights Reserved |