Magnetic Therapy
Below is a list of our references and abstracts concerning magnetic therapy.
_________________________________________________________________
Phys Med Rehabil Clin N Am 1998 Aug;9(3):659-74
Bioelectromagnetic applications for multiple sclerosis.
Richards TL, Lappin MS, Lawrie FW, Stegbauer KC
Department of Radiology, University of Washington, Seattle, USA.
There are EM effects on biology that are potentially both harmful and beneficial. We have reviewed applications of EM fields that are relevant to MS. It is possible that EM fields could be developed into a reproducible therapy for both symptom management and long-term care for MS. The long-term care for MS would have to include beneficial changes in the immune system and in nerve regeneration.
_________________________
Phys Med Rehabil Clin N Am 1999 Aug;10(3):729-54
Evolution of magnetic therapy from alternative to traditional medicine.
Vallbona C, Richards T
Department of Family and Community Medicine, Baylor College of Medicine, Houston, Texas, USA.
Static or electromagnetic fields have been used for centuries to control pain and other biologic problems, but scientific evidence of their effect had not been gathered until recently. This article explores the value of magnetic therapy in rehabilitation medicine in terms of static magnetic fields and time varying magnetic fields (electromagnetic). A historical review is given and the discussion covers the areas of scientific criteria, modalities of magnetic therapy, mechanisms of the biologic effects of magnetic fields, and perspectives on the future of magnetic therapy.
_________________________
J Altern Complement Med 1997 Spring;3(1):21-9
Published erratum appears in J Altern Complement Med 1997 Summer;3(2):205
Double-blind study of pulsing magnetic field effects on multiple sclerosis.
Richards TL, Lappin MS, Acosta-Urquidi J, Kraft GH, Heide AC, Lawrie FW, Merrill TE, Melton GB, Cunningham CA
Department of Radiology, University of Washington, Seattle, USA.
We performed a double-blind study to measure the clinical and subclinical effects of an alternative medicine magnetic device on disease activity in multiple sclerosis (MS). The MS patients were exposed to a magnetic pulsing device (Enermed) where the frequency of the magnetic pulse was in the 4-13 Hz range (50-100 milliGauss). A total of 30 MS patients wore the device on preselected sites between 10 and 24 hours a day for 2 months. Half of the patients (15) randomly received an Enermed device that was magnetically inactive and the other half received an active device. Each MS patient received a set of tests to evaluate MS disease status before and after wearing the Enermed device. The tests included (1) a clinical rating (Kurtzke, EDSS), (2) patient-reported performance scales, and (3) quantitative electroencephalography (QEEG) during a language task. Although there was no significant change between pretreatment and posttreatment in the EDSS scale, there was a significant improvement in the performance scale (PS) combined rating for bladder control, cognitive function, fatigue level, mobility, spasticity, and vision (active group -3.83 +/- 1.08, p < 0.005; placebo group -0.17 +/- 1.07, change in PS scale). There was also a significant change between pretreatment and posttreatment in alpha EEG magnitude during the language task recorded at various electrode sites on the left side. In this double-blind, placebo-controlled study, we have demonstrated a statistically significant effect of the Enermed magnetic pulsing device on patient performance scales and on alpha EEG magnitude during a language task.
_________________________
,In Biologic Effects of Light 1998 Symposium, Eds Holick MF, Jung EG, Kluwer Academic Publishers: Boston, pg. 337-342, 1999
Pulsing magnetic field effects on brain electrical activity in multiple sclerosis
Richards TL, Acosta-Urquidi, J
Multiple sclerosis (MS) is a disease of the central nervous system. Clinical symptoms include central fatigue, impaired bladder control, muscle weakness, sensory deficits, impaired cognition, and others. The cause of MS is unknown, but from histologic, immunologic, and radiologic studies, we know that there are demyelinated brain lesions (visible on magnetic resonance images) that contain immune cells such as macrophages and T-cells (visible on microscopic analysis of brain sections) Recently, a histologic study has also shown that widespread axonal damage occurs in MS along with demyelination. What is the possible connection between MS and bioelectromagnetic fields? We recently published a review entitled "Bioelectromagnetic applications for multiple sclerosis," which examined several scientific studies that demonstrated the effects of electromagnetic fields on nerve regeneration, brain electrical activity (electroencephalography), neurochemistry, and immune system components. All of these effects are important for disease pathology and clinical symptoms in multiple sclerosis (MS). EEG was measured in this study in order to test our hypothesis that the pulsing magnetic device affects the brain electrical activity, and that this may be a mechanism for the effect we have observed on patient-reported symptoms. The EEG data reported previously were measured only during resting and language conditions. The purpose of the current study was to measure the effect of the magnetic device on EEG activity during and after photic stimulation with flashing lights. After photic stimulation, there was a statistically significant increase in alpha EEG magnitude that was greater in the active group compared to the placebo group in electrode positions P3, T5, and O1 (analysis of variance p<.001, F=14, DF = 1,16). In the comparison between active versus placebo, changes measured from three electrode positions were statistically significantly even after multiple comparison correction.
___________________________________________________________________
EVALUATION OF A PULSED-MAGNETIC FIELD DEVICE ON
MULTIPLE SCLEROSIS SYMPTOMS
Poster presented at the Annual Meeting of the Consortium of Multiple Sclerosis Centers, October 2-4, 1998, Cleveland, Ohio
TL Richards, PhD, University of Washington, Radiology Department
MS Lappin, PhD, Energy Medicine Developments (North America) Inc.
ED Kramer, MD, Cooper Health System/ Robert Wood Johnson, Medical School
Camden, NJ
AD Alquist, University of Washington, Radiology Department
BE Floyd, University of Washington, Radiology Department
KC Stegbauer, , University of Washington, Radiology Department
FW Lawrie, Energy Medicine Developments (North America) Inc.
Introduction
The primary purpose of this research was to test the effects of a pulsing electromagnetic field on the symptoms and quality of life experienced by individuals suffering from multiple sclerosis (MS). This project used the Enermed device (Energy Medicine Developments (North America), Inc.) to expose patients to individually programmed pulsed electromagnetic fields. Previous results using this device have been reported for both multiple sclerosis (1) and migraine patients (2). We have recently published a review of bioelectromagnetic applications for multiple sclerosis (3) and this document contains background information on bioelectromagnetism and possible mechanisms for biological effects. Weak electromagnetic fields have been shown to affect nerve regeneration, brain electrical activity (electroencephalography), neurochemistry, and immune system components (see reference 3 and citations therein).
Methods
Study design
The effectiveness of the treatment was assessed in a 10 week, randomized, placebo-controlled, double-blind crossover study. Data were collected from three sites: University of Washington, Seattle, WA; Cooper Health System/Robert Wood Johnson Medical School, Camden, NJ; Neurology Center of Fairfax, Virginia. Subjects came on site for 4 visits for evaluation before and after exposure to both active (Enermed) and inactive (Placebo) devices. This study was reviewed and approved by the Institutional Review Board/ Human Subjects Committee at each site.
MS Subjects
MS patients were recruited from all three sites (125 total completed the study). To meet inclusion criteria subjects were: 1) diagnosed with clinically definite MS (Poser criteria); 2) between the ages of 18 to 60 inclusive; 3) medically stable -- no MS exacerbations for two months prior to the study; 4) medicinally stable - no changes in prescription for one month prior; 5) disabled in bladder control or muscle spasticity with a symptom rating of 2 or higher (0 = no symptoms, 5 = severe symptoms).
The Pulsing Magnetic Field Exposure
Subjects were exposed to a pulsing magnetic field produced by the Enermed device. This device is a watch-sized, programmable magnet powered by a 3 volt battery. It is designed to be worn close to the body, and produces a 1 millisecond magnetic pulse in the 50 to 100 milliGauss range. A programmable chip allows the magnetic pulse trains to be sequentially produced for one to 16 frequencies (12.7 seconds duration for each frequency). In order to determine the most appropriate frequencies for treatment, each patient was scanned with a Bioelectric Frequency Analyzer (BFA) which measures weak electromagnetic fields produced by the body. The BFA consists of a quartz crystal detector embedded in a padded headset. The output signal is linked to an amplifier and a small computer for spectrum analysis. After subjects' devices were programmed they were shown how to tape or attach the device to a spot just below the collar bone on the chest. They were instructed to wear the device 6 hours the first day and then increase wearing time by 2 hours each day up to 24 hours a day.
Outcome Measures
The primary outcomes of this study were daily diary measures of bladder control and muscle spasticity. These included reports of daytime and nighttime urination frequency and ratings of the severity of any spasticity experienced during the day and night. Daily reports for the last three weeks of each treatment session (Enermed and Placebo) were averaged and compared. Secondary outcomes consisted of symptom ratings scales from the MS Quality of Life Inventory (MSQLI) developed by the Health Services Research Subcommittee of the Consortium of Multiple Sclerosis Centers (4). We used four of the abbreviated MSQLI scales (bladder control, fatigue, perceived deficits, and pain/sensory problems), plus a spasticity scale we created by modifying the pain effects scale. The surveys were administered prior to the first treatment session (baseline measure), and immediately following each four week treatment session. All five scales had excellent internal consistency reliabilities with Cronbach alphas averaging .80 or higher across the three time periods.
Results
Daily Diary Data
The daily diary measures were analyzed using repeated measures ANOVA, both with and without adjusting for covariates (age, sex, ability to walk 10 yards unassisted, and treatment sequence). Neither analysis showed significant treatment effects on frequency of urination or daily spasticity ratings.
Survey Data
Sample Selection. Before analyzing the survey data we dropped the 5 subjects who had physician diagnosed exacerbations during the course of the study and the 14 subjects who reported at baseline that they had no problems ("0") with spasticity and bladder control. This allowed us to examine the data for the subset of subjects (n=105) who might truly be candidates for the therapy, i.e., those experiencing relevant symptoms, without the error variance introduced by exacerbations and steroid treatments.
Spasticity and Bladder Control Problems. The means on the spasticity and bladder control scales favored the Enermed group, but were not statistically significant.
Fatigue, Pain, and Cognitive Problems. Next we looked at the remaining three MSQLI scales: fatigue, pain/sensory effects, and perceived cognitive deficits. We were especially interested in fatigue, as this symptom showed the greatest improvement within the Enermed group in our first study (1). Paired t-test results showed a significant treatment effect for fatigue (t=-2.59, p<.01), but not for the other two symptoms (Pain t=-1.30, p<.20; Cogntive t=-.66, p<.51) . To rule out the possibility that the observed effects for fatigue were due to one or two extreme scores in the negative tail of the distribution, we also conducted a non-parametric test (Wilcoxon Signed Ranks test), based on ranks instead of means. This test also showed a significant treatment effect. In fact, ranks from the Wilcoxon test indicate that the ratio of subjects showing improvement on the Enermed relative to the placebo device was about 1.6 to 1.0 (56:36 with 12 ties). In other words, for every 10 subjects showing greater placebo effects , 16 subjects showed greater improvements on the Enermed device.
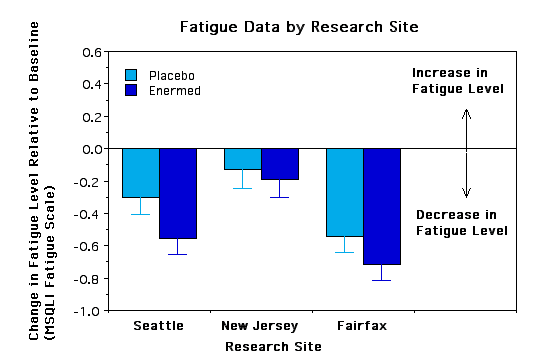
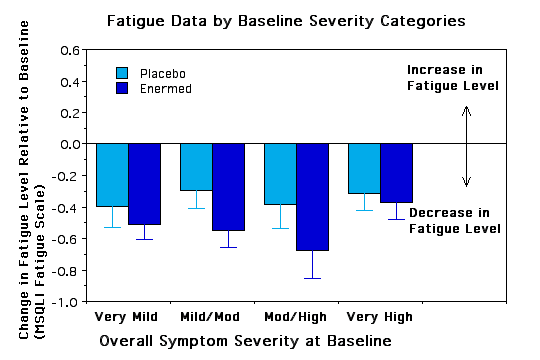
Subgroup Analyses. To see if certain subgroups responded differently to the Enermed we used repeated measures ANOVA to examine fatigue as a function of a) years with MS, b) ability to walk 10 yards, c) severity of symptoms at baseline, d) type of MS, e) sex, and f) research site. None of the interactions was significant, however, there were some trends worth noting.. The Enermed appears to be most effective at alleviating fatigue for MS patients who can walk 10 yards unassisted and whose symptoms are neither very mild nor very extreme (see Figures 3 and 4). There were also unexpected significant main effects for research site - overall changes were greater at the Fairfax site than the New Jersey site, although the treatment effects appear proportional at the two sites (see Figure 5). Possible reasons for these differences need to be explored further.
Summary and Conclusion
Although the primary outcome measures (dairy reports) did not show significant treatment effects, the results of our secondary, survey-based analyses were encouraging. The MSQLI fatigue scale showed a significant treatment effect, and post-hoc analyses showed this result was replicated in one of the two single item measures of fatigue included in other instruments. These results clearly warrant additional research with a primary focus on fatigue. Fatigue is one of the most debilitating symptoms associated with MS, and any non-invasive, non-pharmacological therapy that might alleviate its effects would be a tremendous addition to the therapies currently available to MS patients.
References:
1. Richards TL, Lappin MS, Acosta-Urquidi J, Kraft GH, Heide AC, Lawrie FW, Merrill TE, Melton GB, Cunningham CA, Double-blind study of pulsing magnetic field effects on multiple sclerosis. J Altern Complement Med 3:21, 1997.
2. Lappin MS, Research on the utility of medigen device as a treatment for migraines. Research Report #1, April 1995. Vancouver, B. C.: Energy Medicine Developments (North America) Inc.
3. Richards TL, Lappin MS, Lawrie FW, Stegbauer KC, Bioelectromagnetic applications for multiple sclerosis, Physical Medicine and Rehabilitation Clinics of North America, 9, 659-674, 1998.
4. Ritvo PG, Fischer JS, Miller DM, Andrews H, Paty DW, LaRocca NG, Multiple Sclerosis Quality of Life Inventory: A User's Manual. New York: National Multiple Sclerosis Society, 1997.
Acknowledgements
We thank the Multiple Sclerosis Association of America for their generous financial support. We also thank Dr. Richard Kronmall and Statistics and Epidemiology Researach Corporation for help with statistics and data analysis. We acknowledge the help of Catherine Cunningham for recruitment of subjects.
Figure 1 is a real Enermed Device. - placed next to methods
Figure 2 is a diagram of placement of Enermed Device on the body.
Figure 3 - placed below results section

Figure 4
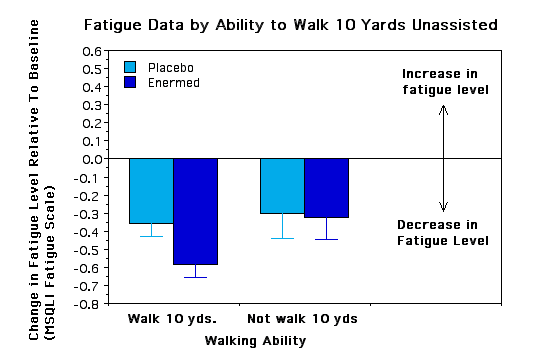
Figure 5