American Journal of Neuroradiology, 21:916-922, May 2000
(with written permission from AJNR to post on this web site)
MS#01346-99
The Effects of a Phonologically-Driven Treatment for Dyslexia on Lactate Levels as Measured by Proton MRSI
Todd L. Richards, David Corina, Sandra Serafini, Keith Steury, Denise R. Echelard, Stephen R. Dager, Ken Marro, Robert D. Abbott, Kenneth R. Maravilla, Virginia W. Berninger
From the Departments Radiology (T.L.R., S.R.D., K.M., K.R.M.), Psychiatry and Behavioral Science (S.R.D.), Psychology (D.C., K.S.), Speech and Hearing Sciences (S.S.), Bioengineering (T.L.R., S.R.D.), College of Education (R.D.A., V.W.B.), University of Washington, Seattle
Grant support: This work was funded by a special multidisciplinary learning disabilities Center Grant from NIH (NICHD), P50 HD33812. The authors thank Drs. Martin Kushmerick and James Nelson for their encouragement and support of this study.
Corresponding author: Todd L. Richards, Radiology Department, Box 357115, University of Washington, Seattle, Wa. 98195 USA
e-mail address: toddr@u.washington.edu
Phone: 206-548-6725 Fax: 206-543-3495
Abstract
Background and Purpose
: Dyslexia is a language disorder in which reading ability is compromised due to poor phonological skills. The purpose of this study was to measure the effect of a phonologically-driven treatment for dyslexia on brain lactate response to language stimulation as measured by proton MR spectroscopic imaging.Methods: Brain lactate metabolism was measured at two different time points (1 year apart) during four different cognitive tasks (3 language tasks and 1 non-language task) in dyslexic boys(n=8) and in control boys(n=7) using a fast MRSI technique called proton echo-planar spectroscopic imaging (1 cm3 voxel resolution). The age range for both dyslexics and controls was 10-13 years old. In between the first and second imaging session, the dyslexic boys participated in an instructional intervention which was a reading/science workshop.
Results: Before treatment, the dyslexic boys showed significantly greater lactate elevation compared to a control group in the left anterior quadrant (analysis of variance, p=.05) of the brain during a phonological task. However, after treatment brain lactate elevation was not significantly different from controls in the left anterior quadrant during the same phonological task. Behaviorally, the dyslexic children improved in phonological aspects of reading.
Conclusion: Instructional intervention that improved phonological performance in dyslexic boys was associated with changes in brain lactate levels as measured by proton echo-planar spectroscopic imaging.
Introduction
We have previously reported that dyslexic boys demonstrated a greater area of brain lactate elevation compared to a control group during a phonological task in the left anterior quadrant of the brain (1). Dyslexia is a language disorder characterized by poor reading due to a phonological deficit (1,2). We used a fast spectroscopic imaging technique called proton echo-planar spectroscopic imaging (PEPSI) to non-invasively measure brain lactate changes associated with language activation. Functional MR spectroscopy (fMRS) using the PEPSI technique is an alternative approach for detecting regional brain activation and measures tissue-based lactate changes (a direct measure of metabolism) produced by a temporary mismatch of oxygen delivery and consumption in response to neuronal activation (3). Following completion of the prior fMRS study, the dyslexic boys entered into a treatment program designed to improve their phonological abilities. Both the dyslexic boys and the control boys were re-imaged a year after the initial imaging session. We hypothesized that a positive response to therapy would be reflected by changes in the distribution of brain lactate activation as measured by fMRS. The purpose of this study was to test the effect of this instructionally-based treatment for dyslexia on the brain lactate response during reading-related language tasks.
Materials and Methods
Study Design
Eight dyslexic and seven non-dyslexic (control) boys were imaged using PEPSI (4) while performing four different cognitive tasks. Only males were chosen for this study in order to minimize the variance between subjects, especially given that Shaywitz et al have shown large functional MR imaging (fMRI) differences between the sexes during language processing(5). The dyslexic boys were imaged before and after a three week (plus followups) phonologically-driven treatment for dyslexia described by Berninger (6). The control group was also imaged at the same two time points as the dyslexic group but did not receive treatment because the control boys were already reading well above grade-level and age expectations. However, reimaging the controls at the same time as the dyslexics were reimaged permitted evaluation of where any change in the dyslexics might be due to maturation rather than instruction. The scan protocol and the language stimuli were identical across repeated scans and were equally spaced for the two groups. The boys were not re-imaged immediately after treatment because we wanted to evaluate whether the behavioral gain observed immediately after treatment would result in a long lasting change in lactate brain activity. The dyslexic and control groups were well-matched in age, IQ, and in head-size (number of total voxels) but not in reading skills on which they demonstrated marked differences at the first time point, as described below. The experimental tasks were designed to activate phonological and lexical access functions of the brain, while a tone task was used to activate auditory non-language functions of the brain. Two control tasks (listening to scanner noise and passive listening of word lists) were used to subtract out low-level acoustic stimulus effects. The phonological and lexical access tasks engage additional linguistic processes beyond that required for passive listening of stimulus words. Task specific activation was assessed by subtracting out the passive listening condition from the phonological and lexical access tasks. This method provides a means to identify activation related to processing phonological information or accessing word meanings, independent of brain activation due to the characteristics of word stimuli,. The component of brain activation related to auditory processing not specific to language was assessed by subtracting out the scanner noise from the tone judgments.
Magnetic Resonance Imaging and Spectroscopy
Conventional MR imaging and PEPSI were performed on a clinical 1.5 Tesla Signa MR imaging system (General Electric, Waukesha) equipped with version 5.7 software and a custom-built radiofrequency (RF) head coil developed by Hayes et al.(7). MR images were acquired in the sagittal plane (TR/TE 600/20 msec) and also in the axial plane (TR/TE1/TE2 2000/35/80 msec). The custom designed head coil was necessary to acquire MR spectroscopic data with high enough signal-to-noise ratio to detect the small lactate peak and also to maintain a reasonably short acquisition time to avoid motion and habituation artifacts from these young subjects. This custom rf head coil has been measured at producing an MR signal with 35% better signal-to-noise than the standard GE head rf coil (product hardware delivered with the scanner) (7). The coordinates of the Sylvian fissure and surrounding language-related structures were determined on the sagittal images which were co-registered with the axial images for both MR imaging and spectroscopic imaging. The areas sampled with PEPSI were based on the work of Ojemann et al. (8) that invasively demonstrated language activation in the anatomic region encompassing the Sylvian fissure and adjacent opercula. The single PEPSI slice was oriented to encompass the frontal operculum and the posterior portion of the superior temporal gyrus. Deeper subcortical structures were also included that are associated (through neuronal connectivity) with the cortical areas. Proton spectra were acquired using PEPSI, a spin-echo pulse sequence developed by Posse et al. (4) that allows fast spectroscopic imaging which is 32 time faster than conventional hydrogen spectroscopic imaging for the same spatial resolution. PEPSI is a method that is somewhat demanding on gradient amplifiers and may not function properly on most conventional scanners without echo-planar capability. PEPSI was chosen in our experiment over a single-voxel technique such as PRESS because we needed to map the lactate distribution over the whole brain slice in order to define the regional metabolic activation. Parameters for data acquisition included: TR/TE 4000/272 msec; 2 averages; 32 x 16 spatial matrix (zero-filled to 32x32 for reconstruction); 512 echoes in the echo-planar acquisition; 32 complex points per echo; full echo acquisition; field of view 24 cm; and slice thickness 20 mm. Voxel size was approximately 1 cm3. and the acquisition time for each PEPSI scan was approximately 4 1/2 minutes. Five PEPSI scans were acquired at each session during: 1) baseline (no extra sounds); 2) phonological task; 3) lexical access task; 4) tone discrimination task; and 5) passive listening as described below. Data were processed as described previously (9). The metabolites were integrated using the following procedure: 1) magnetic field inhomogeneity shifts (B0 shifts) were corrected by finding the maximum point of the NAA peak and resetting the ppm scale to 2.0 ppm for each spectrum; 2) the average baseline was determined from 32 points to the right of 0.0 ppm; 3) the maximum intensity point of the peak was determined within a set spectral range ( NAA = 2.0 +/- 0.07, lactate = 1.3 +/- 0.1 ppm); and 4) integration was performed by summing the spectral intensities for the NAA and lactate for the ppm ranges specified in step 3.
Subject Characterization
The University of Washington Human Subjects Institutional Review Board approval was obtained for this study, and each subject (as well as parent/guardian) gave written, informed consent. All subjects were right handed (90-100% on the Edinburgh Handedness scale(10)). The control boys had a history of learning to read easily and were reading above normal for age (average was one standard deviation above mean for age using the Woodcock Reading Mastery Test-Revised (11)) . The dyslexic boys had a developmental history of extreme difficulty in learning to read despite many forms of extra assistance at school and also had a family history of multi-generational dyslexia, which was confirmed in a concurrent family genetics study (W. Raskind, personal communication) at our center. At the initial scan, the dyslexic boys were reading on average 1.66 standard deviations below the mean for age on the Woodcock test (11). In addition, all the dyslexic boys were shown to have deficits in three skills that predict ease of learning to read and response to intervention: phonological (phoneme segmentation and/or memory for spoken nonwords), rapid automatized naming, and orthographic (speed of coding written words and/or accuracy of representing them in memory)(12) . Based on independent t-tests, the controls ( M=127.3, SD=10.8) and dyslexics (M=124.3, SD=11.1) did not differ in age in months at the time of the initial scan (t(11) = 0.49,p=0.637). Likewise, at the time of the initial scan, the controls (M=15.6, SD=3.2) and dyslexics (M=13.2, SD=1.6) did not differ in age-corrected WISC-III vocabulary scores (t (11)= 1.68, p=0.12), which provide the best estimate of Full Scale IQ. However, at the time of the initial scan, the controls and dyslexics did differ significantly in age-corrected standard scores for reading real words on the Word Identification (WI) subtest of the Woodcock Reading Mastery Test-Revised (WRMT-R) and for reading pseudowords on the Word Attack (WA) subtest of the WRMT-R: t(11)=6.81, p < 0.001 on the WI subtest and t(10) = 6.02, p<0.001 on the WA subtest. The differences for both real word reading (WI, controls, M=115.1, SD=9.2; dyslexics, M=75.5, SD=11.8) and pseudoword reading (WA, controls, M=110.2, SD=6.8; dyslexics, M=79.0, SD=10.7) were large as well as statistically significant.
Phonologically-Driven Instructional Treatment
Treatment consisted of fifteen 2-hour group sessions in a 3-week reading/science workshop. To motivate the boys who had a long struggle in learning to read, they were told that they were Einstein's Ninja Turtles. The instructor told them the story of how Albert Einstein had had learning disabilities in reading that he overcame (13). She also explained that gene mutations can confer special advantages, like the Ninja turtles, as well as disadvantages, like trouble learning to read. She used the fable of the tortoise and the hare to convince them that despite a slow start in learning to read, they could finish the race as skilled readers. A systems approach to intervention was used in the treatment in which instruction was aimed at all levels of language (subword, word, and text, (6)) during the first hour of each instructional session. Each session began with sound games to remediate their deficits in phonological processing. High-interest, polysyllabic words taken from science texts were presented orally. The boys counted the number of syllables in the spoken word and used colored counters to represent each phoneme in the syllables. Only after they analyzed the phonological structure of each word did they see the same words in written form. Next they were taught to decode the words using syllable patterns of written English that derive from the Anglo-Saxon, Latin/Romance, and Greek origins of English words (14) and correspondences between 1- and 2- letter spelling units and phonemes (12). Then, they competed in a "reading bee" to decode transfer words that varied in predictability and complexity but contained the most common syllable patterns and spelling-phoneme correspondences of English. Finally, they took turns reading orally science texts on the human brain (week 1), astronomy (week 2) and endangered species (week 3). Following this phonologically-driven instruction, the last hour of each session was devoted to hands-on science activities that contained the words practiced in the beginning of the session. Guest speakers who were scientists came to give lectures and information about famous scientists as part of this training. Follow-up sessions over several months were held to facilitate and maintain the improvements. Additional procedural details are reported by Berninger(6).
Brain Stimulation Tasks During PEPSI Scanning
During repeated MR scanning, the same brain stimulation tasks were used. The children were asked to listen to aurally-presented words, non-words, and tone pairs at a rate of one stimulus pair every 4 sec. Language stimuli were composed of four groupings of word pairs, crossed for lexical status (word vs. non-word) and sound similarity (rhyming vs. non-rhyming) resulting in four sets of stimuli: word/word:nonrhyming (e.g. FLY-CHURCH); word/word:rhyming (FLY-EYE), word/nonword:nonrhyming (CROW-TREEL); word/non-word:rhyming (MEAL-TREEL). Nonwords such as TREEL allow assessment of sound processing without any meaning cues. The presenting order of word pair-types were counterbalanced and thus the ordering effects were controlled for. During rhyming (phonological task), subjects listened to the word pairs and judged whether they rhymed or did not rhyme; whether words were real was irrelevant. During the lexical access condition, subjects listened to the same word pairs, and judged whether the word pairs contained two real words, or contained a non-word; whether or not the words rhymed was irrelevant. Thus same stimulus lists were used for lexical access and rhyming, only the task instructions were changed. Subjects indicated their rhyme and lexical decisions by raising cards held in the right and left hands (the hand used to signal a "yes" response was counterbalanced across subjects). During passive listening, subjects listened to the same stimuli, but were instructed to alternately raise the left and right hand without making any judgments on the stimuli. For the tone judgment task, five pure tones (329.6hz, 350hz, 415.3hz, 440hz, & 523hz), were grouped into pairs of identical tones, or different tones. Subjects were asked to raise one hand if the tones were identical and to raise the opposite hand if the tones were different. The subjects were tested for accuracy of their responses for all tasks during a prescan training session and during the actual MR scanning. For the tone subtraction, the baseline scanner noise was used instead of passive listening. The PEPSI acquisition time for each task for approximately 4 1/2 minutes and a recovery period of 5 minutes was allowed between tasks based in part on the time for lactate recovery measured by Frahm et al (3).
Data Analysis
To affirmatively evaluate focal brain activation from PEPSI images, Z-score maps were created from the lactate/NAA ratios based on the following equation:
[lactate/NAA (task) - lactate/NAA (passive listening)] / [standard deviation of lactate/NAA (passive listening
)], where (task) in the equation refers to the task given during the scan which was either phonological, lexical access, or tone differentiation. The standard deviation of the lactate/NAA was calculated for each subject using all valid spectra of the control task (either passive listening or scanner noise). This Z-score was calculated for all voxels that contained valid spectra for each language condition(9). Definition of the threshold for lactate elevation indicating brain activation was based on Z-scores greater than 2.0 on a voxel by voxel basis. The cross-sectional PEPSI slice of the brain was divided into 4 quadrants (described below) and the number of voxels with elevated lactate within each quadrant was counted for each subject.The PEPSI data were analyzed to sum the number of activated voxels (with elevated lactate above the threshold) in each of the four regions of the brain . Because regional specificity of lactate response is not well established and also because of the large variability between subjects in the spatial location of the lactate response, we divided the spectroscopic imaging slice into four quadrants. The brain was divided into four quadrants based on 1) left to right -- brain midline defined on the axial MR image and 2) anterior to posterior -- using the midpoint of the thalamus as a landmark. Inferential statistics were used to compare relative activation for each group in each brain quadrant on each task. ANOVA was used to test for differences in the number of activated voxels between controls and dyslexics. The number of valid voxels for the dyslexic group was not significantly different from that of the control group (controls, M=160.6, SD=6.9; dyslexics, M=166, SD=19, t (11) = 0.76, p<.48).
The lactate/NAA ratio was used in order to normalize for radiofrequency inhomogeneity, variable cerebrospinal fluid contribution, and also to standardize the lactate signal across subjects. An automated computer-software mask was applied to the spectra to ensure that the MR lactate signal was not contaminated with scalp lipid signal
(1). In this software, a true lactate peak was differentiated from lipid contamination based on MR frequency which was determined from an in vitro lactate measurement (the lipid peak MR frequency was not the same as lactate frequency). Additionally, in previous work, we varied the TE to assess J-coupling properties of the peak at 1.3 ppm which identified this peak to be lactate (15).Results
Behavioral Improvement
Immediately following the intervention, the boys improved, on average, 8.9 points on age-corrected standard scores (0.6 standard deviation unit) of a measure of phonological decoding (WA WRMT-R (11)). On a criterion measure of oral reading of text, three were at the grade level just completed, three were above the grade level just completed, and one was still below grade level for oral reading but was at the grade level just completed for silent reading. Eight months later (about a year following the initial MRI and PEPSI neuroimaging scans), all but two had age-appropriate phonological awareness, the group showed a relative gain of 0.9 standard score units in phonological memory since the initial assessment prior to the first imaging session, and the group maintained a relative gain of 8.7 points on age-corrected standard scores (0.6 standard deviation unit) on phonological decoding since first assessed prior to neuroimaging (Table 1). Their phonological decoding (word attack) was now at the border between low average and average (M=89.7, SD=8.5 (6)). Additional details are reported by Berninger
(6).MR spectroscopic imaging changes
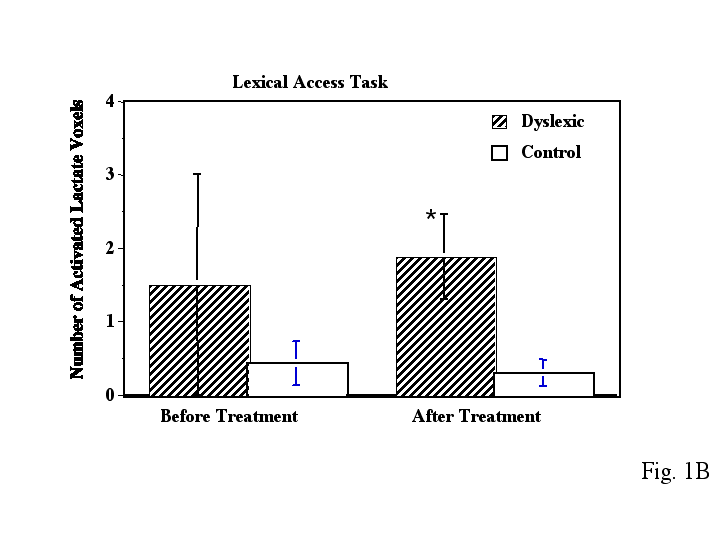
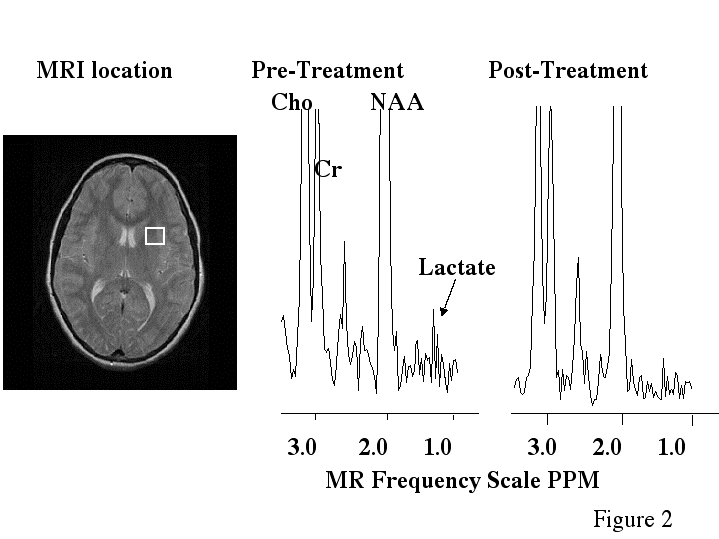
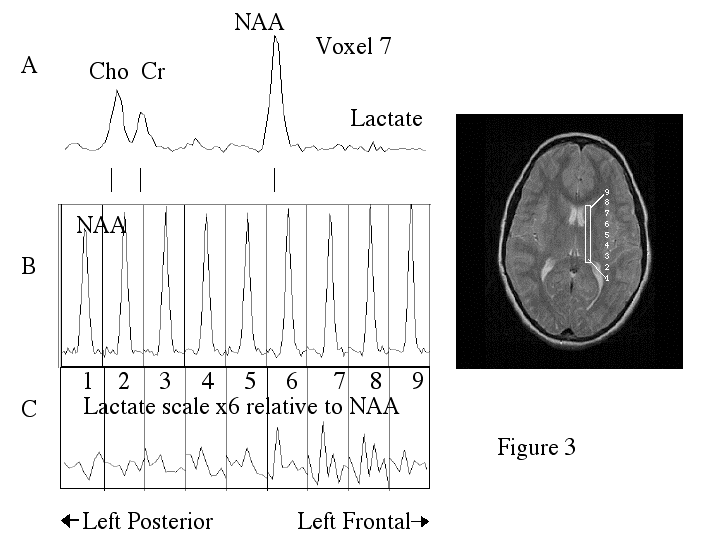
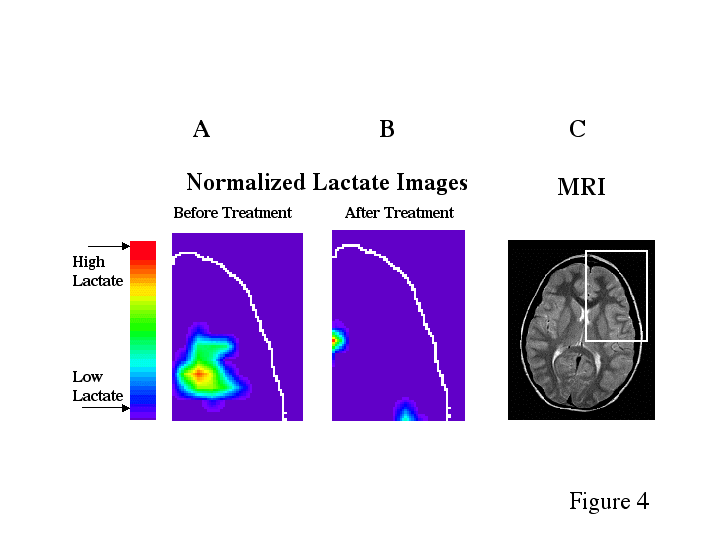
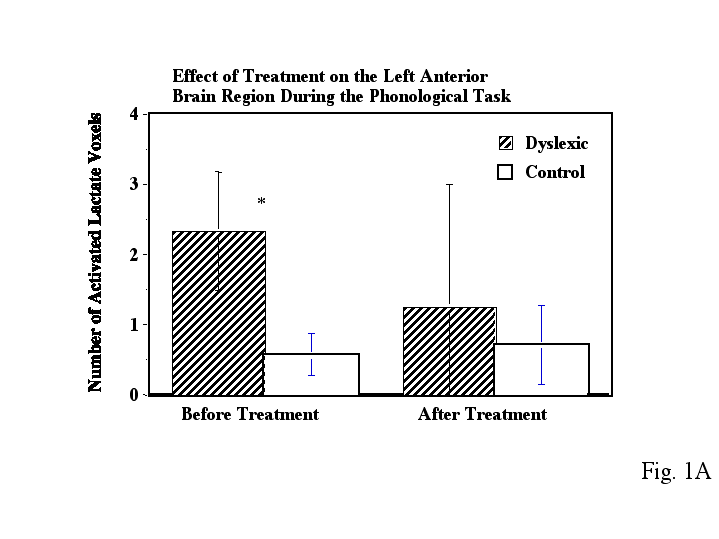
Before treatment, dyslexic boys had significantly more brain voxels with elevated MR lactate levels (2.33 +/-SE 0.843) compared to a control group (0.57 +/- SE 0.30) during a phonological task in the left anterior quadrant (Table 2, ANOVA, F(1,11)= 4.41, p=.05). However after treatment, for the same phonological task, and in the same region of the brain, the dyslexic boys were not significantly different from controls, who were stable across repeated imaging (Table 2, Fig. 1A). Figure 2 is an example of spectra demonstrating the reduction in lactate from a dyslexic subject before and after treatment in the left anterior brain region during the phonological task . Figure 3 is example of spectra from a dyslexic boy during the phonological task demonstrating increased lactate in the mid-frontal region of the brain compared to the posterior portion. Figure 4 is an example of normalized lactate metabolite images of the left-frontal region of the brain from a dyslexic boy before and after treatment. For the lexical access task, although there was no significant difference between dyslexics and controls before treatment, there was a significant difference after treatment in the left anterior brain region (Table 2, Fig. 1B). Again, the controls were stable across repeated imaging during the lexical access task (Table 2, Fig 1B). Examining the pre and post treatment data indicates that at each time point dyslexics had overall elevated rates for the lexical access task. However the variability observed for this task during the first scanning session likely obscured the significance of this effect. For the lexical access task, the intervention had reduced the variance in this dyslexic group as can be seen in Fig 1A.
As can be seen in Figure 1 and Table 2, the control boys showed very consistent activated voxel counts at the two time points for both the phonological and lexical access tasks. For the tone task, there was no significant difference between dyslexics and controls at any time point (either before or after treatment).
Discussion
Our main finding was that after treatment, the dyslexic children had a reduction in the regional distribution of metabolic activation, characterized by the number of voxels with elevated lactate in the left anterior quadrant during the phonological task. This reduction may be an indication that the treatment was effective in reducing the amount of lactate produced during metabolic activation required to perform phonological judgments. Concurrently collected behavioral data verified that the subjects were attentively listening to the auditory stimuli. The behavioral data also demonstrated that the dyslexic children were less accurate than the control children for the phonological task prior to treatment. The left anterior quadrant where we observed this change after treatment is an area that encompasses portions of the frontal operculum, inferior frontal gyrus, and anterior temporal lobe - areas associated with motor speech. It also encompasses portions of the frontal lobe that are known to be associated with executive functioning of the brain (16). Our findings suggest that these areas are involved with dyslexia and also that treatment is affecting lactate metabolism in these areas. The fact that control averages for the number of activated voxels remained quite constant across the treatment period supports the validity of the observed change in the dyslexic children. At the same time lactate metabolic activation increased during the lexical access task for the dyslexics compared to controls. Apparently, once these boys were phonologically aware, it was more difficult for them to attend to meaning but ignore phonology when they were asked to judge between real and non-real words. Despite the reduction in lactate activation during the phonological task following phonological training, a brain signature may remain in which some linguistic processes are still difficult for dyslexics.
Though the present measure is not well suited for highly resolved spatial localization, it does provide new evidence for lactate metabolic changes following behavioral intervention. Importantly, this technique permits assessment of neuronal activation changes that are physiologically different than is currently obtained from imaging studies reliant upon hemodynamic properties (as measured by fMRI). Thus, the present study adds to a growing number of studies that have demonstrated functional imaging changes associated with behavioral changes or reorganization of the brain after recovery from language deficits(17,18).
Conclusions
Our findings are important because they show that treatment is accompanied by lactate changes in the brain as well as changes in performance on behavioral tasks in young children with a developmental rather than an acquired disorder. This change was in the direction of a decreased area of lactate activation. However, we recognize that lactate changes can be interpreted by a number of different mechanisms including alterations in blood blow or mitochondrial dysfunction. The change in lactate metabolism may be a brain substrate for functional verbal efficiency (19) which is decreased in poor readers to some degree but may increase following partial remediation. Our results are also important because they suggest that the brain is not only an independent variable that can cause a language disorder such as dyslexia but is also a dependent variable that can be modified by instructional intervention from the environment (20).
Figure Legends:
Figure 1 - Bar graph of number of average activated voxels (as defined by MRS lactate increases) in the left anterior brain quadrant for both dyslexic and control children during the A) phonological task and B) lexical access task. The left side data is before treatment and the right side is after treatment. Error bars are standard error of the mean. The * asterisk indicates dyslexic versus control comparisons that were significantly different. The data was collected using proton echo-planar spectroscopic imaging (PEPSI) with parameters TR/TE 4000/272 msec.
Figure 2 - MR image and proton spectra from an activated brain region of one dyslexic subject.
a, MR image with white box indicating the brain region measured with MR spectroscopy. b, proton MR spectrum from the white box brain region before treatment (the lactate resonance has a signal-to-noise ratio of 2.2). c, proton MR spectrum from the white box brain region after treatment (lactate has a signal-to-noise of 1.6) . The spectroscopic data was collected using proton echo-planar spectroscopic imaging (PEPSI) with parameters TR/TE 4000/272 msec. The MR image was collected using the fast spin-echo pulse sequence and parameters TR/TE 2000/80 msec. The intensity axis of the spectra is scaled so that the lactate can be easier visualized; however, Cho and NAA are scaled off the figure. Notice the decrease in the lactate peaks for the after treatment spectrum. Abbreviations: Cho - choline, CR - creatine, NAA - N-acetyl aspartate.
Figure 3
Proton MR spectra and image from a dyslexic boy before treatment during the phonological task.
A) Spectrum from voxel # 7 showing choline (CHO), creatine (Cr), N-acetyl aspartate (NAA), and lactate.
B) Nine spectra from nine adjacent voxels (position shown in part D) zoomed into the NAA region (1.7 to 2.3 ppm).
C) Nine spectra from nine adjacent voxels zoomed into the lactate region (1.10 to 1.33 ppm). The vertical scale of this set was increased 6 times higher than the NAA set so that lactate could be easily visualized. An increase in lactate can be clearly seen in voxels 6 to 8 compared to voxels 1-4.
D) The MR image shows the location of the 9 voxels shown in parts A-C.
Figure 4
A and B) Normalized lactate images from the left anterior quadrant of the slice shown in the MR image of a dyslexic boy created from the PEPSI spectra before and after the phonologically-driven instructional treatment. The data was collected using proton echo-planar spectroscopic imaging (PEPSI) with parameters TR/TE 4000/272 msec.
C) MR image shows the middle slide of the PEPSI anatomical slice location. The white box on the MR image shows the area that is displayed in the lactate images. This is the brain region that had a significant difference between dyslexics and controls. The lactate was normalized according to this equation: (lactate/NAA phono.)- (lactate/NAA passive).
References
1. Richards TL, Dager SR, Corina D, et al. Dyslexic children have abnormal brain lactate response to reading-related language tasks. AJNR, Amer J Neurorad 1999; 20:1393-1398
2. Wagner R and Torgesen J. The nature of phonological processing and its role in the acquisition of reading skills. Psychological Bulletin 1987; 101:192-212
3. Frahm J, Krueger G, Merboldt KD, and Kleinschmidt A. Dynamic NMR studies of perfusion and oxidative metabolism during focal brain activation. Adv Exp Med Biol 1997; 413:195-203
4. Posse S, Dager SR, Richards TL, et al. In Vivo Measurement of Regional Brain Metabolic Response to Hyperventilation using Magnetic Resonance Proton Echo Planar Spectroscopic Imaging (PEPSI). Mag Res Med 1997; 37:858-865
5. Shaywitz BA, Shaywitz SE, Pugh KR, et al. Sex differences in the functional organization of the brain for language [see comments]. Nature 1995; 373:607-9
6. Berninger VW. Dyslexia: the invisible, treatable disorder: The story of Einstein's ninja turtles. Learning Disabilities Quarterly 2000; in press
7. Hayes CE and Mathis CM. Improved brain coil for fMRI and high resolution imaging, Fourth Annual Meeting of the Society of Magnetic Resonance, Berkeley. 1996
8. Ojemann G. Localization of language in frontal cortex. Adv Neurol 1992; 57:361-8
9. Richards TL, Dager SR, Panagiotides HS, et al. Functional MR Spectroscopy During Language Activation, a preliminary study using proton echo-planar spectroscopic imaging (PEPSI). Int J Neurorad 1997; 3:490-495
10. Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia 1971; 9:97-113
11. Woodcock R. Woodcock reading mastery test - revised, Minnesota MN: American Guidance Service. 1987
12. Berninger V. Process Assesement of the Learner: Guides for Intervention, San Antonio, TX: The Psychological Corporation. 1998
13. Patten B. Visual mediated thinking: A report of the case of Albert Einstein. J Learn Dis 1973; 6:15-20
14. Henry M. Words: Integrated decoding and spelling instruction based on word origin and word structure, Austin: Pro-Ed. 1990
15. Dager SR, Marro KI, Peterson J, and Richards TL. Applications of Magnetic Resonance Spectroscopy to Investigate Panic Disorder. In: H. Nasrallah and J. Pettegrew, eds. NMR Spectroscopy in Psychiatric Brain Disorders. Washington DC: American Psychiatric Press, Inc. 1995:147-177
16. Leskela M, Hietanen M, Kalska H, Ylikoski R, Phjasvaara T, Mantyla R, Erkinjuntti T. Executive functions and speed of mental processing in elderly patients with frontal or nonfrontal ischemic stroke. Eur J Neurol 1999; 6:653-661
17. Small SL, Flores DK, and Noll DC. Different neural circuits subserve reading before and after therapy for acquired dyslexia. Brain Lang 1998; 62:298-308
18. Thulborn KR, Carpenter PA, and Just MA. Plasticity of language-related brain function during recovery from stroke. Stroke 1999; 30:749-54
19. Perfetti C. Reading Ability, New York: Oxford University Press. 1985
20. Berninger VW and Corina D. Making cognitive neuroscience educationally relevant: Creating bi-directional collaborations between educational psychology and cognitive neuroscience. Educational Psychology Review 1998; 10:343-354
Table 1. Phonological Skills of the Dyslexic Boys
Measures 1st Scan Rx 1 Rx 2
Phonological Decodinga 81.0+9.8 89.9+9.6 89.7+8.5
Phonological Memoryb -0.62+0.67 na 0.28+1.05
Phonological Awarenessc all below grade na all but 2 at or
above grade
Notes:
All numbers are mean + standard deviation
a— age-corrected standard score on WRMT-R word attack (11) with mean = 100, SD=
15.
b— grade-corrected z-score with mean = 0, SD = 1 from Wagner & Torgesen,
Comprehensive Tests of Phonological Awareness, Pro-Ed, Austin, Texas, 1999.
c— Deletion task criterion reference for grade.
Rx 1 refers to measurements made immediately after treatment.
Rx 2 refers to measurements made at the time of the second neuroimaging scan which was approximately 1 year after the first scan.
Table 2. Activate Lactate Voxel Counts for the Left Anterior Brain Region
Phonological Task
Treatment Status Condition Lactate Voxel Count
Before Treatment Controls 0.57 +/- 0.30
After Treatment Controls 0.71 +/- 0.56
Before Treatment Dyslexics 2.33 +/- 0.84 **(p<.05)
After Treatment Dyslexics 1.25 +/- 0.62
Lexical Access Task
Treatment Status Condition Lactate Voxel Count
Before Treatment Controls 0.43 +/- 0.30
After Treatment Controls 0.29 +/- 0.18
Before Treatment Dyslexics 1.50 +/- 1.50
After Treatment Dyslexics 1.88 +/- 0.58 ** (p<.05)
Note: The lactate voxel count is expressed as average +/- standard error.