Genetic engineering for cardiac fibrosis
The Davis Lab uses both in vitro and in vivo genetic engineering techniques to target the fibrotic response and better understand the cellular and molecular underpinnings of cardiac wound healing. Present in virtually every mode of cardiac disease, fibrosis in the heart is both a hemodynamic burden and a strong prognostic indicator of heart failure. Due to the limited regenerative capacity of the myocardium, the heart’s response to injury is to permanently scar creating a myocardial environment that is altogether more hostile to regeneration. Through the use of genome-wide functional screens for cellular differentiation we have started to identify novel molecular and mechanical signaling networks that program cells to create fibrotic tissue. Our ultimate objective is to leverage these networks as a means of developing interventions directed at enhancing repair and/or mitigating the fibrotic response. As many of these signaling networks are likely part of the global injury response, our lab can utilize these genetic engineering approaches to further investigate the injury response in other tissues including skeletal muscle, lung, skin, and even tumor metastasis.
Intercepting fibrotic networks for cardiac regeneration
The Davis lab is focused on uncovering the mechanistic basis for how the heart heals, repairs, and remodels in response to injury and disease. Toward this end we are tackling a fundamental problem associated with every form of heart disease, which is the replacement of contractile muscle with fibrotic scarring. The presence of fibrotic scar impairs both repair and function of the heart and rapidly accelerates the disease process. We’ve been implementing genome-wide screening and genetic engineering of cells and mice to resolve the signaling networks causal for fibrotic scar formation. Our ultimate goal is to leverage this molecular network to tactically block or regress scar formation in patients thereby slowing the disease process and creating an environment more amenable to regenerative therapies.
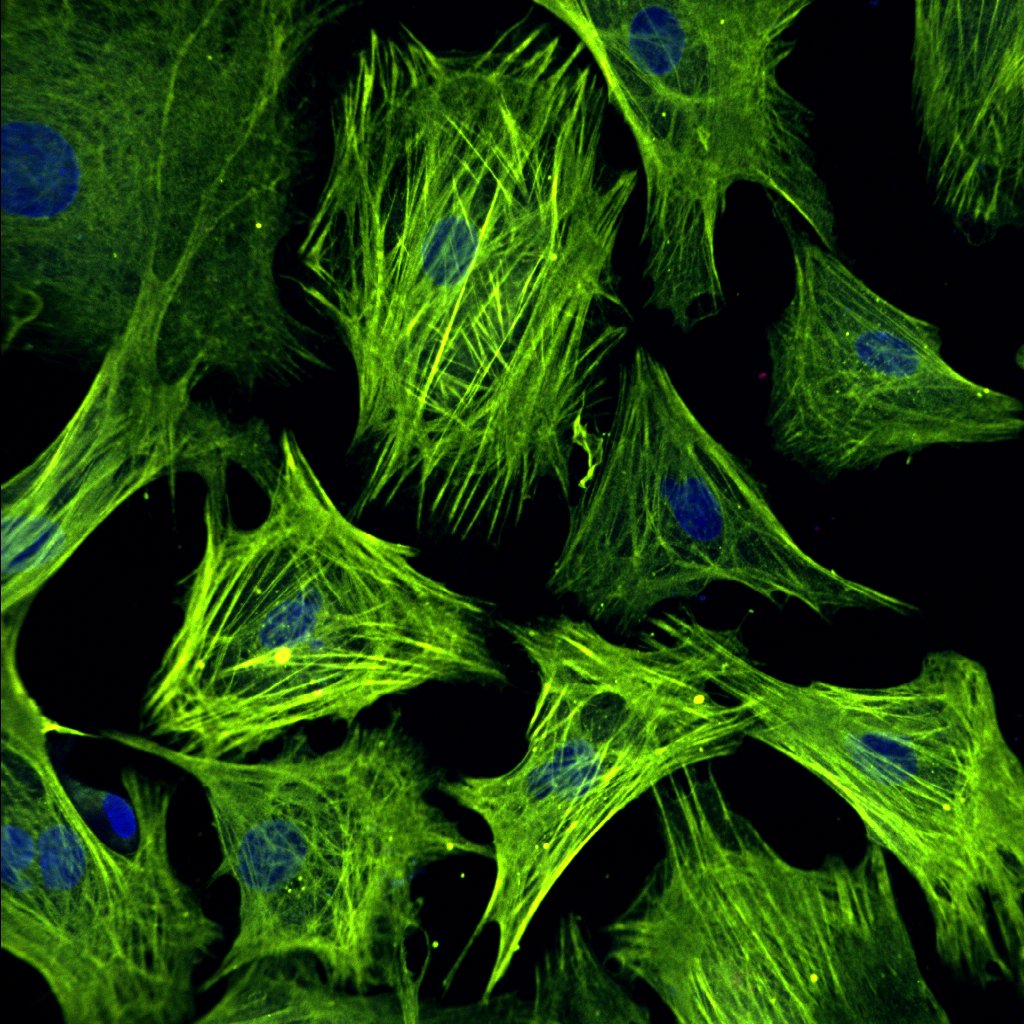
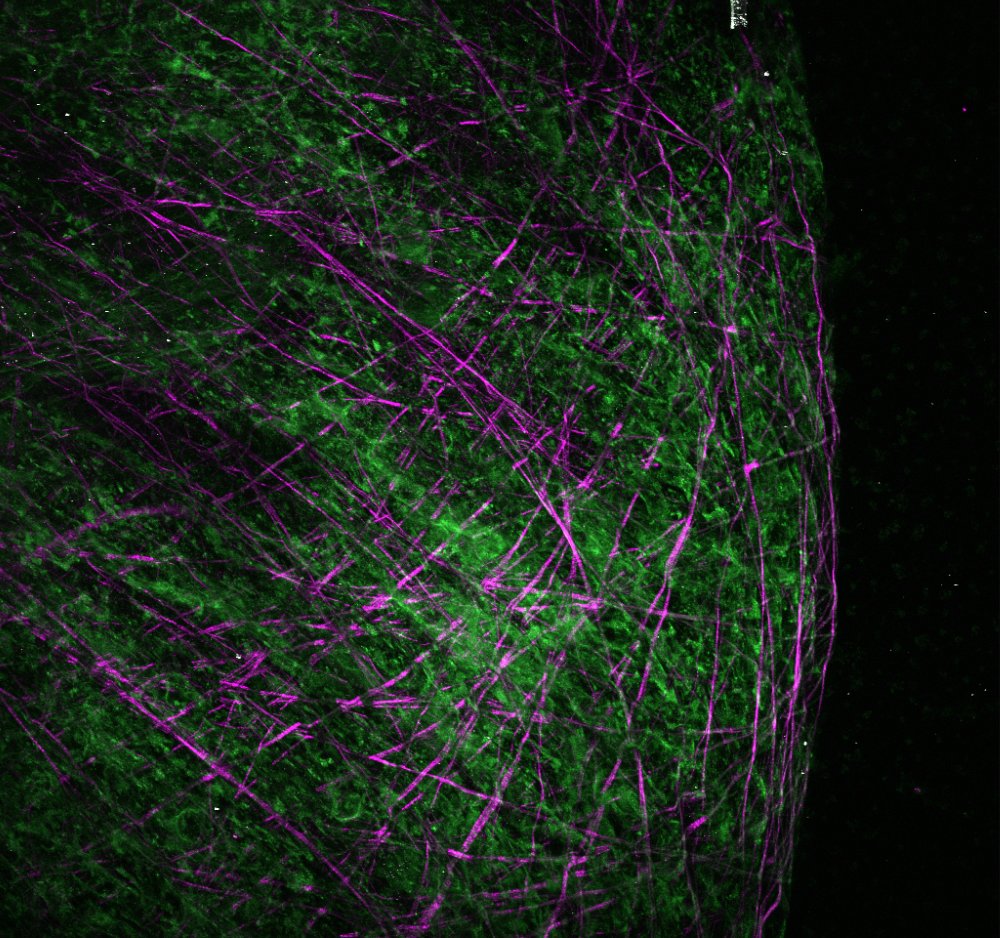
Uncovering the mechanical underpinnings of cardiac remodeling
Equally problematic drivers of heart disease are compensatory changes in cardiac architecture. Recently, we demonstrated that biomechanical signals are central determinants of the nature and severity of the heart’s size and shape. In lieu of this result, our lab seeks to understand how cell and tissue forces are sensed and transduced into changes in cell geometry, differentiation, and proliferation. Given that mechanical forces are more easily manipulated and induce quicker responses than genetic and biochemical strategies, we anticipate this work pioneer new mechanics-based therapeutics that reverse maladaptive cardiac architecture back to normal.

Our Team
Led by principal investigator Jennifer Davis, the lab brings together scientists and clinical collaborators from a diverse array of fields, including pathology, bioengineering, molecular biology, physiology, chemical engineering, and more…
Contact
Phone :
Email :
Address :
Jennifer Davis
Bioengineering; University of Washington
SLU – 344 Brotman
Box 358050
Seattle, WA 98195-8050