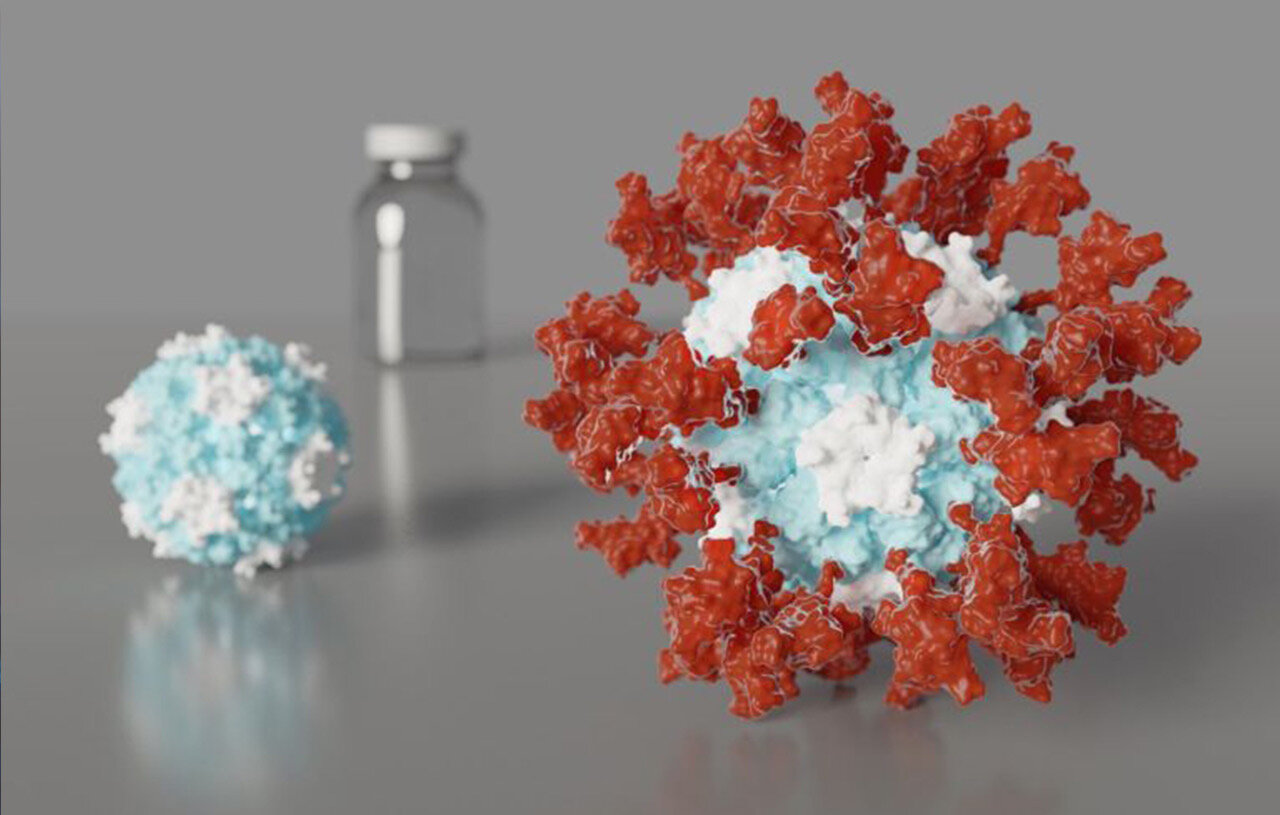
We identified ACE2 as the SARS-CoV-2 receptor and unveiled the architecture of the viral spike (infection machinery)
Recent Work
We designed a COVID-19 protein subunit vaccine currently evaluated in clinical trials
We discovered a variant-proof human antibody neutralizing SARS-CoV-2 and SARS-CoV used as therapeutic
SARS-CoV and MERS-CoV neutralizing antibody structures reveal a mechanism of receptor-functional mimicry
We obtained the first atomic-level description of a coronavirus spike glycoprotein trimer before and after viral entry
We are always looking for bright people that are eager to work with us. Please contact David directly by e-mail at dveesler@uw.edu or using the link below.