Lutz Lab
Department of Bioengineering, University of
Washington
Home Research Publications People News Contact
Current Research
Paper-based point-of-care diagnostics for low-resource settings [link]
 In
joint work with Paul Yager and Elain
Fu in UW Bioengineering, we are using the wicking action of paper to create
inexpensive and easy to use device for disease diagnostics. The devices are
similar to pregnancy tests, but we use shaped paper and built-in timing
mechanisms to “program” paper to carry out the steps normally done in a
laboratory by a trained human or a fancy machine. Examples include automating
multi-step biochemical tests such as sample preparation and signal
amplification. We currently have large projects with collaborators from PATH,
UW Medicine, Epoch Biosciences, and GE Global Research. Visit the link above
for more information, and visit our Microfluidics 2.0
website (www.mf20.org).
In
joint work with Paul Yager and Elain
Fu in UW Bioengineering, we are using the wicking action of paper to create
inexpensive and easy to use device for disease diagnostics. The devices are
similar to pregnancy tests, but we use shaped paper and built-in timing
mechanisms to “program” paper to carry out the steps normally done in a
laboratory by a trained human or a fancy machine. Examples include automating
multi-step biochemical tests such as sample preparation and signal
amplification. We currently have large projects with collaborators from PATH,
UW Medicine, Epoch Biosciences, and GE Global Research. Visit the link above
for more information, and visit our Microfluidics 2.0
website (www.mf20.org).
Oscillating flow microfluidics (“AC microfluidics”) for point-of-care diagnostics [link]
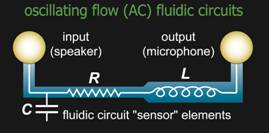 Most
microfluidic devices continue to rely on bulky and
expensive supporting equipment, such as pumps, controllers, and detection
instruments. Nearly everyone on the planet carries a device with sophisticated
control and sensing functions: a cell phone. In this work we are exploiting the
analogy between electrical circuits and fluidic systems to create point-of-care
diagnostics controlled by the audio signals from a cell phone.
Most
microfluidic devices continue to rely on bulky and
expensive supporting equipment, such as pumps, controllers, and detection
instruments. Nearly everyone on the planet carries a device with sophisticated
control and sensing functions: a cell phone. In this work we are exploiting the
analogy between electrical circuits and fluidic systems to create point-of-care
diagnostics controlled by the audio signals from a cell phone.
Simplified assays for low-resource laboratories [link]
 Most of my work in diagnostics focuses on design for
the lowest resource settings, but many more people in the developing world can
be served in the short term by modest regional laboratories with moderate
equipment and training. We are working to reconfigure assay chemistries so that
cumbersome and lengthy laboratory tests can be done quickly with less training
and equipment.
Most of my work in diagnostics focuses on design for
the lowest resource settings, but many more people in the developing world can
be served in the short term by modest regional laboratories with moderate
equipment and training. We are working to reconfigure assay chemistries so that
cumbersome and lengthy laboratory tests can be done quickly with less training
and equipment.
Development of a smart fluidic devices to treat hydrocephalus [link]
 Hydrocephalus
is the inability to drain cerebrospinal fluid (CSF). It occurs in 1 in 500 live
births, and if untreated normally results in death. In the 1950’s, the
hydrocephalus shunt was introduced, which is a permanently implanted drainage
tube and valve that drains fluid from the brain to location in the body where
it can be reabsorbed (e.g., the abdomen). Shunts are life-saving devices, but
they have a notorious failure rate: 40% of shunts fail by two years and 98%
fail by 10 years. We are developing several technologies that aim to reduce
shunt failure and provide more accurate CSF control. This work is a partnership
with my friend Sam Browd, a Pediatric Neurosurgeon
and UW Faculty. My contribution is applying fluidic control principles designed
to address the problems that Sam faces every day.
Hydrocephalus
is the inability to drain cerebrospinal fluid (CSF). It occurs in 1 in 500 live
births, and if untreated normally results in death. In the 1950’s, the
hydrocephalus shunt was introduced, which is a permanently implanted drainage
tube and valve that drains fluid from the brain to location in the body where
it can be reabsorbed (e.g., the abdomen). Shunts are life-saving devices, but
they have a notorious failure rate: 40% of shunts fail by two years and 98%
fail by 10 years. We are developing several technologies that aim to reduce
shunt failure and provide more accurate CSF control. This work is a partnership
with my friend Sam Browd, a Pediatric Neurosurgeon
and UW Faculty. My contribution is applying fluidic control principles designed
to address the problems that Sam faces every day.
Past Research
Raman nanoparticle probes for multiplexed protein detection (prior work with Intel & Beatrice Knudsen, FHCRC) [link]
 Fluorescent probes are
limited in the number that can be differentiated (multiplexed) since the colors
overlap. Raman nanoparticle probes are a new class of
optical labels based on surface-enhanced Raman emission that can give bright
signals with unique spectral fingerprints well-suited for multiplexing. The
Intel team developed composite organic inorganic nanoparticles
(COINs) coupled to antibodies, and I worked on-site at the Fred Hutchinson
Cancer Research Center (FHCRC) to develop COIN applications for multiplexed
protein detection in cancer tissue samples. As part of this work, we developed
a simple spectral fitting algorithm that is easy to implement, allows deconvolution of multiplexed Raman probe signals, allows
removal of unknown background signals (e.g., tissue autofluorescence),
and provides point-by-point estimates of error from multiplexed images.
Fluorescent probes are
limited in the number that can be differentiated (multiplexed) since the colors
overlap. Raman nanoparticle probes are a new class of
optical labels based on surface-enhanced Raman emission that can give bright
signals with unique spectral fingerprints well-suited for multiplexing. The
Intel team developed composite organic inorganic nanoparticles
(COINs) coupled to antibodies, and I worked on-site at the Fred Hutchinson
Cancer Research Center (FHCRC) to develop COIN applications for multiplexed
protein detection in cancer tissue samples. As part of this work, we developed
a simple spectral fitting algorithm that is easy to implement, allows deconvolution of multiplexed Raman probe signals, allows
removal of unknown background signals (e.g., tissue autofluorescence),
and provides point-by-point estimates of error from multiplexed images.
Oscillating flow microfluidics for single cell trapping (prior work with Dan Schwartz, UW) [link]
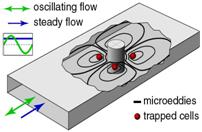 When
oscillating fluid is required to turn around an obstacle or a bend, it creates
a secondary flow that usually involves circulating eddies. Theory was
established as far back as the 19th century by Lord Rayleigh, but it
has mostly been a curiosity in fluid dynamics literature. In my PhD work with
Dan Schwartz, we found that this odd flow creates fluid forces that trap single
cells without any physical contact. Trap forces are strong enough to hold
swimming cells (we trapped swimming plankton) and to hold cells while flowing
fluid past them (at very high rates). Devices are easy to build and can be
driven by a home stereo amplifier. I remain interested in this area but have no
current projects, but Dan has an ongoing effort (link).
When
oscillating fluid is required to turn around an obstacle or a bend, it creates
a secondary flow that usually involves circulating eddies. Theory was
established as far back as the 19th century by Lord Rayleigh, but it
has mostly been a curiosity in fluid dynamics literature. In my PhD work with
Dan Schwartz, we found that this odd flow creates fluid forces that trap single
cells without any physical contact. Trap forces are strong enough to hold
swimming cells (we trapped swimming plankton) and to hold cells while flowing
fluid past them (at very high rates). Devices are easy to build and can be
driven by a home stereo amplifier. I remain interested in this area but have no
current projects, but Dan has an ongoing effort (link).