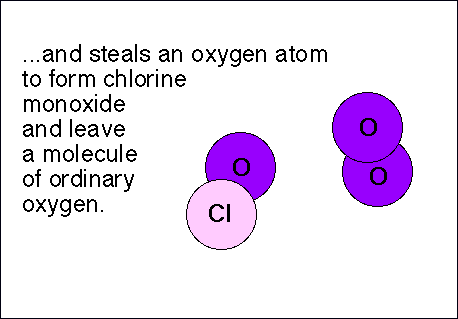The Ozone Hole
Ozone is a colorless gas which consists of three oxygen atoms, one double-bonded to another, and the third loosely bound with a single bond to one of the other two. It is a highly unstable molecule, and will take any opportunity to break apart to form a more stable oxygen atom and a reactive oxygen radical. It naturally exists mostly in the stratosphere, where it acts as a thin chemical shield against solar ultraviolet radiation, but it is produced at ground level by any process which involves an electrical current passing through the air (the "dry" odor you smell when you're next to a copy machine is ozone, for instance) Ground level ozone has caused serious problems, especially in recent decades as more and more of it is produced by our growing population. It reacts with a variety of materials, including the rubber in automobile tires, and the tissues lining the lung, to name a couple.
In the stratosphere, however, ozone absorbs ultraviolet light via the process described below, effectively removing a significant portion of harmful high-energy electromagnetic radiation from the spectrum which reaches us at ground level. Exposure to high levels of UV light has been proven to increase the risk of malignant skin cancers and melanoma, as well as affecting the fragile balance in natural ecosystems. Therefore, any reduction in stratospheric ozone concentration may result in increased incidence of skin cancers and other UV related maladies.
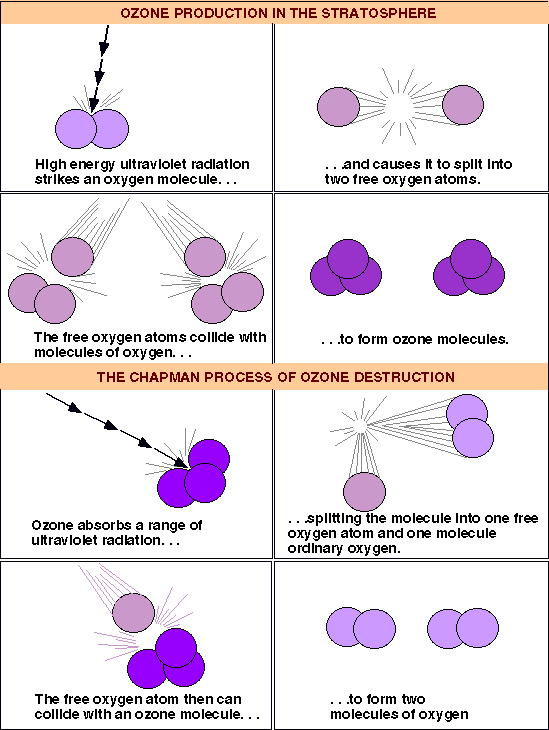 Here is the process by which stratospheric ozone is created. A molecule of oxygen is hit by a ray of ultraviolet radiation. This gives it enough energy to split into two oxygen radicals, which go on to hit other oxygen molecules, bonding with them to form ozone. Later, ozone molecules are struck by ultraviolet radiation and break apart into oxygen molecules and oxygen radicals, to continue the cycle.
Here is the process by which stratospheric ozone is created. A molecule of oxygen is hit by a ray of ultraviolet radiation. This gives it enough energy to split into two oxygen radicals, which go on to hit other oxygen molecules, bonding with them to form ozone. Later, ozone molecules are struck by ultraviolet radiation and break apart into oxygen molecules and oxygen radicals, to continue the cycle.
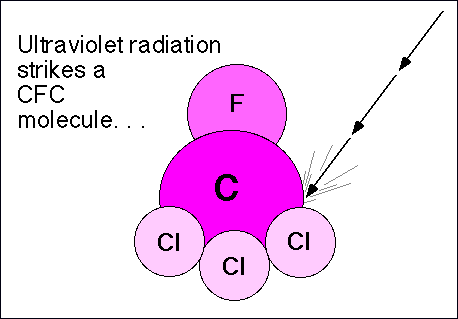
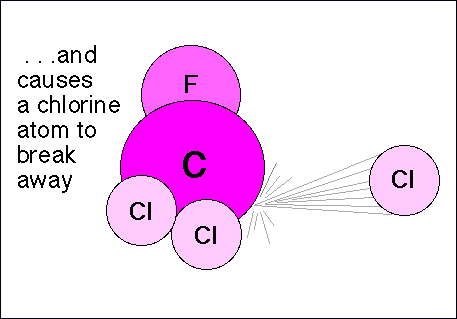
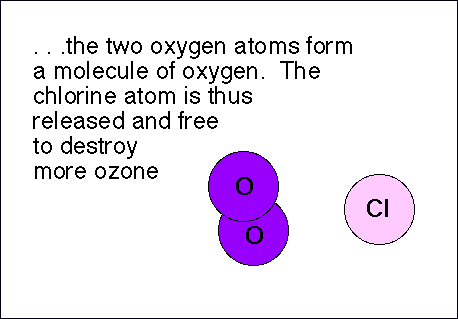
Chlorofluorocarbons (CFCs) are relatively stable compounds used as refrigerants, and used to be utilized to blow-mold Styrofoam. These compounds are affected by ultraviolet radiation and pose a threat to the ozone layer in the following manner:

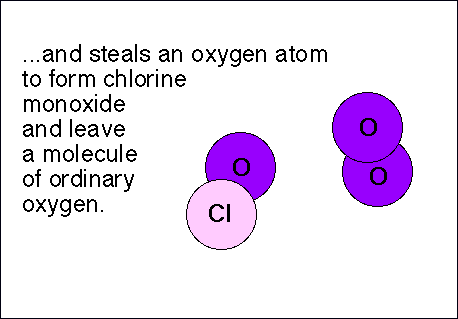


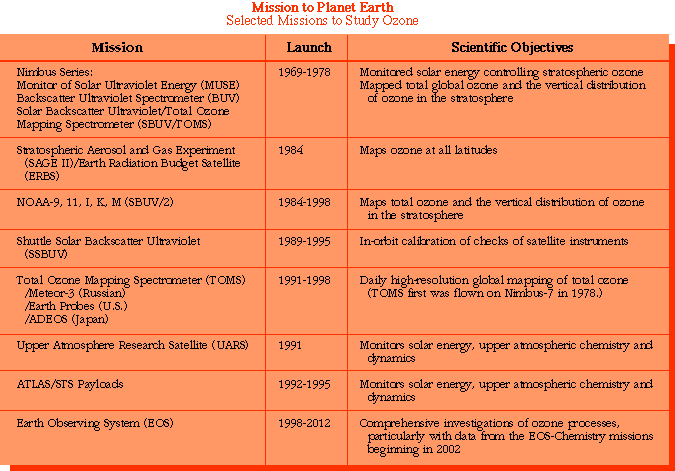
Here is a list of some of the ways we've used to study ozone and other atmospheric compounds:
Back to the list of talk subjects!!
Back to the Science Outreach Home Page!!
 Here is the process by which stratospheric ozone is created. A molecule of oxygen is hit by a ray of ultraviolet radiation. This gives it enough energy to split into two oxygen radicals, which go on to hit other oxygen molecules, bonding with them to form ozone. Later, ozone molecules are struck by ultraviolet radiation and break apart into oxygen molecules and oxygen radicals, to continue the cycle.
Here is the process by which stratospheric ozone is created. A molecule of oxygen is hit by a ray of ultraviolet radiation. This gives it enough energy to split into two oxygen radicals, which go on to hit other oxygen molecules, bonding with them to form ozone. Later, ozone molecules are struck by ultraviolet radiation and break apart into oxygen molecules and oxygen radicals, to continue the cycle.