 |
Bioengineering Department, Box 355061, University of Washington, Seattle, WA 98195, USA |
 |
 |
Bioengineering Department, Box 355061, University of Washington, Seattle, WA 98195, USA |
 |
Note: This report summarizes activity in the first 9 months of funding (August 1, 1995 to April 30, 1996).
Delivering therapeutic drugs to the intended site of action at a controlled rate is an important goal of modern medicine. It is now well understood that controlling the rate of release of drugs can have several positive effects on the long-term outcome for the patient. Recent work has centered on fabrication of macroscopic devices that allow continuous release of some drugs under limited circumstances. We have begun a novel approach to continuous release in which neither macroscopic matrix nor pump is required, and the "device fabrication" is accomplished through molecular-level self-assembly. The long-term objective of the work is to develop a device-free method by which many drugs can be released into the body in a continuous manner (0th-order kinetics) through association with self-assembling tubular microstructures.
Lipid tubules are a recently discovered self-organizing system in which lipids crystallize into tightly packed bilayers that spontaneously form hollow cylinders less than 1 µm in diameter. Because of the tight packing of the surfactants in tubules, the microstructures should dissolve (or be enzymatically degraded) from their ends only. Since the size of the end (the only available surface area for removal of drug) is constant until the tubule is annihilated, a population of tubules of uniform length will release surfactant at a constant rate. Since the wide range of two-chain surfactants now known to form tubules includes lipidated polypeptides, and such lipidated polypeptides are composed of innocuous nontoxic biomolecules, it should be possible to synthesize tubule-forming lipids with polar headgroups that contain polypeptide and non-polypeptide drugs. These lipidated drugs might be active themselves if the hydrophobic groups do not interfere with binding at their sites of action; alternatively, the tubule-forming drugs might function as prodrugs that become active when their hydrophobic moieties are cleaved. The aforementioned tight packing of the lipids will be transferred to the drug headgroups. Such structures will either dissolve spontaneously at a rate controlled by structure-determined solubility of the lipidated prodrug molecules, or they might release drug by enzymatic degradation of the microstructures from their ends. These "prodrug tubules" could function as continuous release systems independent of any macroscopic encapsulation or delivery devices.
Tubule-based continuous release would probably have first applications in topical administration of drugs, antibacterials and antifungals, release of antitumor drugs/peptides by injections of tubules into or near tumors, antibiotic and growth factor delivery for wound dressings, long-acting vaccines, delivery of peptide and non-peptide drugs from subcutaneous sites, and in vitro use for tissue/cell culture factors.
The short-term objective of this project is to perform in vitro experiments that prove or disprove the following 3 hypotheses:
A homogeneous population of lipid tubules will dissolve (or be enzymatically degraded) in such a manner that the rate of release of the constituent molecules (or parts thereof) will be constant until the microstructures are consumed-
We believe that we have demonstrated this for the case of the diacetylenic tubules, with the caveat that the use of PLA2 as the enzyme produces hydrolytic products that are not soluble.
Ligation to an appropriate hydrophobic anchoring moiety will allow both model water-soluble molecules and clinically significant drugs and prodrugs to self-associate into tubular microstructures. Of particular interest are bioactive polypeptides.
We have begun synthesis of a set of peptide-based surfactants, and have made preliminary characterization of several of these.
Tubule-associated drugs or prodrugs will continuously release drugs in simulations of drug delivery environments, either through dissolution of the molecules from tubule ends or through enzymatic cleavage from the tubules.
We have not yet begun tests of this sort.
The Gelb and Yager groups provide complementary expertise in organic synthesis, enzymatic degradation of lipid bilayers and the self-assembly of lipids into tubules. Primary techniques employed are organic synthesis, differential scanning calorimetry, optical and electron microscopies, and Raman spectroscopy. The work to be accomplished will provide the basis for exploitation of this new route of continuous release of drugs by establishing principles for design of tubule-forming lipopeptides and other prodrugs of therapeutic value.
The following numbered tasks were listed in the timeline in the grant as submitted to NIH/Whitaker (see Appendix) and were to be at least initiated during the first year of the program. They are presented in the order in which they appeared in that timeline.
1. Determine hydrolysis kinetics of diacetylenic tubules by PLA2 using FA binding protein assay
To test whether the tubule geometry produces a constant rate of degradation, enzymatic cleavage of lipid tubules was studied in a well-described model system. The commercially available tubule-forming phospholipid 1,2-bis(10,12-tricosadiynoyl)-sn-glycero-3-phosphocholine (DC8,9PC) and the enzyme phospholipase A2 (PLA2) were used as the model system. Upon precipitation from ethanol, DC8,9PC forms multilamellar tubules with an average diameter of 0.75 µm, a length distribution ranging from 30-50 µm, and a melting temperature (Tm) of 43.8 °C. The tubule morphology is composed of helically-wrapped lipid bilayers that close to form straight, hollow, rigid tubes. Tubules often appear, however, in the presence of minority structures such as open helical ribbons. Helical striation patterns on the outside of closed tubules are often observable by electron microscopy, as they are in tubule samples used in this work. If given time to anneal, the lipids form closed and uniform tubules.
A key assumption is that the tight crystalline packing of the tubule wall will prevent any appreciable release of monomeric lipid from the microstructure, and will be too tight to allow enzymatic hydrolysis of the lipid except at regions of defects in the crystalline packing such as must occur at tubule ends. To compare the effects of performing hydrolysis below Tm on a phospholipid system with a radically different microstructure than a tubule, small unilamellar vesicles (SUVs) were prepared from 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC). The Tm of DPPC at 41.3 °C is similar to DC8,9PC, and is only slightly depressed in SUVs. Because they have identical head groups, comparison of hydrolysis of DPPC vesicles and DC8,9PC tubules allows isolation of those effects unique to a tubular microstructure.
The microstructural form into which phospholipids self-assemble strongly influences the kinetics of hydrolysis. Figure 4 shows the progress curves for the hydrolysis of 0.5 mM dispersions of DPPC SUVs and of multilamellar DC8,9PC tubules at 30 °C by 2.24 mg/ml PLA2 as determined by the production of free fatty acid. The hydrolysis progress curve for the control SUV dispersion of DPPC was biphasic, as expected. An initial rapid hydrolysis stage, which ends after roughly 50% of the total lipid has been hydrolyzed, is followed by period of slower, nearly constant hydrolysis. In a unilamellar liposome, only the outermost monolayer is initially accessible to enzyme. The rapid initial hydrolysis rate of 0.88 s-1 reflects the hydrolysis of lipids in the outer monolayer. The onset of the subsequent slower hydrolysis stage is caused by substrate depletion in the outer monolayer. Hydrolysis proceeds to completion at about 0.044 s-1, limited by access to new substrate either from the bursting of partially hydrolyzed vesicles or from slow phospholipid flip-flop between the inner and outer vesicle monolayers.
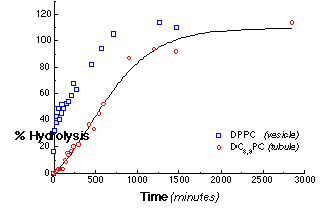
Figure 4 - The total hydrolysis progress curves for suspensions of DC8,9PC and DPPC by PLA2. The concentration of PLA2 was 160 nM (2.24 mg/ml). Each point within the progress curves was determined by the acrylodated-intestinal fatty acid binding protein (ADIFAB) assay for free fatty acid hydrolysis products. This linear release profile was consistent with our model for tubule hydrolysis.
The progress of DC8,9PC tubule hydrolysis is markedly different. After a 120 minute lag, the hydrolysis proceeds with a slow, nearly constant rate of 0.041 s-1 for most of the reaction. The rate of hydrolysis of DC8,9PC tubules after the initial lag is 20 times slower than that for the outermost DPPC vesicle monolayer, and, in contrast to all other reported PLA2 reaction profiles, it remains constant after the initial lag until nearly 100% hydrolysis. This constant hydrolysis rate is consistent with our simple mechanistic model of end-dominated tubule hydrolysis.
If the simple end-driven model of tubule hydrolysis were correct, the extent of hydrolysis with time would be reflected in morphological changes of the lipid microstructures. Our model predicted that a tubule would degrade like a burning cigarette, where its length shortens with time as reaction products leave from the eroding ends. However, the microstructures observed by transmission electron microscopy (TEM) of the digest products, as in Figure 5, reflect a more complex process. Shortly after addition of enzyme, helical ribbons emerging from what appear to be fractured tubules are visible. Even though a few intact tubules are still present at the 50% hydrolysis point, the types of microstructures present include small filaments, helical ribbons, and elongated sheets. Near completion, the reaction mixture contains a rich variety of microstructures; the tubules do not simply shorten from loss of hydrolysis products at the ends.


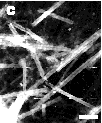
Figure 5 - A time series of negative-stain transmission electron micrographs during the hydrolysis of DC8,9PC lipid micro-structures taken (A) 0 hrs, (B) 1 hr; and (C) 3 hrs after addition of enzyme. Scale bar: 5 µm
The presence of fractured and unwrapped tubules after partial PLA2 hydrolysis suggests that the preferred crystal structure of the hydrolysis products is incompatible with that of the substrate DC8,9PC. Tubules appear to remain intact until a certain fraction of reaction products is reached within a local region of the tubule bilayer. At the point when product accumulation can no longer support the specific asymmetric curvature required to form a one micrometer diameter tubule, the product regions fracture and unwrap to form smaller helices, filaments and flat sheets.
Thermodynamic data obtained from differential scanning calorimetric (DSC) studies of the original lipid tubules and the end-stage microstructures suggest that the hydrolysis products remain within the bilayer. Equimolar mixtures of fatty acids and lyso-phosphatidylcholines are known to form a bilayer even though the individual components form micelles when dispersed in water, and these mixtures have distinct thermotropic transition profiles: they exhibit a twin endothermic transition at a temperature higher than that of the corresponding phosphatidylcholine. DSC heating traces of the end-stage hydrolysis products of DC8,9PC tubules contain a large, broad endotherm at an onset temperature of 69.8°C. The onset of the DC8,9PC reaction product endotherm occurs at a much higher temperature than the Tm of 43.8°C for unhydrolyzed DC8,9PC tubules, which is consistent with the existence of end-product bilayers.
Since tubule hydrolysis was studied in pH 8.5 buffer, the fatty acid reaction products should be at least partially charged in the bilayer. The cationic fluorescent dye, 1,1,3,3,3',3'- hexamethyl-indocarbocyanine iodide (H379) was added to visualize regions of negative charge accumulation. The series of fluorescence and phase-contrast optical micrographs in Figure 6 shows product domain formation within the microstructures. Early in the reaction, fluorescent regions appear at several points along intact tubules. Hydrolysis, therefore, is not limited to tubule ends. If bilayer crystallinity prevents PLA2 hydrolysis except at defects and edges, then the amorphous or defect-rich regions of the bilayer would be more prone to enzymatic attack. Local defects in molecular packing within the bilayer appear to function as initiation sites for hydrolysis. The highly fluorescent helical ribbon, flat sheet, and other small fibrous microstructures seen at later times, like those seen with TEM, must contain a high level of reaction products. Product domains, which form early, originate either from the processive action of PLA2 (i.e., continuous hydrolysis without desorption of enzyme into the aqueous phase) along the surface or from lateral phase separation of reaction products within the bilayer following hydrolysis.

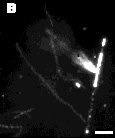

Figure 6 - Digitized phase contrast and fluorescence micrographs showing product domains within the microstructures, as indicated by the cationic fluorescent dye H-379. Images taken (A) 1 hour; (B) 3 hrs; and (D) 10 hrs after the addition of enzyme. Scale bar: 5 µm
Distortions in membrane structure from localized packing defects, phase separation of reaction products, and changes in membrane curvature may lead to recruitment of additional PLA2 to the membrane surface. Enhanced binding of PLA2 to product-rich domains may also affect the hydrolysis profile observed for DC8,9PC tubules. An increase in concentration of enzyme within a localized zone of hydrolysis could accelerate the rate as long as there is adequate access to fresh substrate. To track the distribution of enzyme, 5-carboxyfluorescein-tagged PLA2 was used. Figure 7 shows where PLA2 accumulates on the tubule at the beginning and end of the reaction. Immediately after addition, enzyme distributes uniformly over the tubule surface. By the completion of hydrolysis, the product microstructures (i.e., helical ribbons and filaments) show strong fluorescence, which implies enhanced PLA2 binding to product-rich microstructures. It is unclear, however, whether the accumulation of protein seen within product-rich domains results from recruitment of fresh, active enzyme to the membrane surface.

|
|

|
|
|
|
|
If the molecular disorder, which is intrinsic to a crystalline domain boundary like those found at the tubule ends, is required for PLA2 hydrolysis of gel-phase phospholipid, then increasing the number of ends at a constant phospholipid concentration should increase the hydrolysis rate. Sonication of tubule dispersions at low temperatures fractures the tubules and greatly increases the number of tubule ends, as in Figure 8. The hydrolysis rate for 4 µm-long tubules was twice that for 40 µm-long tubules. Clearly, there is an edge-dependence to the hydrolysis rate, but not to the extent predicted by the change in the number of tubule ends. Sonication may have fractured the tubules in regions already containing a high concentration of molecular packing defects, which would have served as sites for hydrolysis initiation in the original tubules.
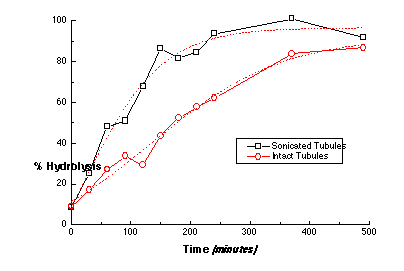
Figure 8 - The hydrolysis profile for two suspensions of DC8,9PC lipid tubules having different mean tubule lengths: (sonicated) 4 µm and (intact) 40 µm as determined by TEM. The reaction buffer (pH 8.5) contained 10 mM Tris-HCl (pH 8.5), 150 mM NaCl, 5 mM CaCl2, 0.01% sodium azide, and 0.5 mM lipid. The concentration of PLA2 in the digest buffer was 160 nM (2.24 mg/ml). Each point within the progress curves was determined by the ADIFAB assay for free fatty acid hydrolysis products. The population having the shorter mean tubule length has a faster rate of hydrolysis, which suggests that the number of ends and edges and defects plays a modest role in hydrolysis.
2. Optical microscopy of hydrolysis of diacetylenic tubules by PLA2 to determine localization of enzymatic activity
see above section
3. Develop working models of molecular structure of tubule-forming lipids
Modeling has been initiated on all lipids synthesized to date using several modeling packages on platforms ranging from the Macintosh to Sun workstations.
4. Determine most accessible points on ceramides and cerebrosides for use as attachment points
see synthesis description below
5. Model effects of alterations in headgroups on bilayer stability using energy minimization of 8x8 arrays
As yet we have not performed full energy minimization on lipid bilayers as large as 8x8. It is questionable whether such calculations are of any value at this point, and preliminary calculations on 3x3 arrays using Chem3D on a series of peptide-headgroup lipids suggest that much larger arrays are probably required for any credible results to be forthcoming. On the other hand, we have found an immediate need to determine the appropriate length of a polypeptide tether that is required to allow trypsin to hydrolyze peptides at the surface of a rigid lipid bilayer. To that end we have extensively modeled the interaction of trypsin and a planar array of Lys-Ala-Sar-Pro3-Glu-(NH-C12)2.
For these studies, a drug lipid consists of three distinct regions: the tubule-forming core (Pro-Glu-(NH-C12)2); an amino acid tether (Sar-Pro2), and an enzymatic cleavage site (Lys-Ala). The peptidic lipid was placed in trypsin's binding pocket by first superimposing the lipid's lys-ala residues over the lys-ala residues of an inhibitor already positioned in the binding pocket. The remaining residues and the hydrocarbon moieties were added so that the lipid would project out orthogonally from the binding pocket. The atomic coordinates for trypsin with the bound peptidic lipid were then added to a monolayer lattice containing a 1:15 ratio of drug lipids and tubule forming core lipids (Pro-Glu-(NH-C12)2). Several dihedral angles (e.g., phi, psi angles) of the amino acids comprising the tether in the trypsin bound lipid were adjusted to minimize steric interactions between the enzyme on the monolayer.
Pictured in Figure 9 is a lattice containing trypsin and a peptidic lipid with a small three-amino-acid-long tether. The enzyme clearly impinges into the monolayer. Results from these simple molecular modeling studies show that a tether segment of at least seven amino acid residues is required. Also determined from these studies is an estimate of the maximum drug loading achievable in a mixed lipid system for trypsin to act interfacially without unfavorable steric interactions with neighboring drug lipids (e.g., 1:15 loading ratio).
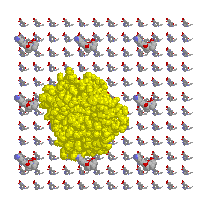
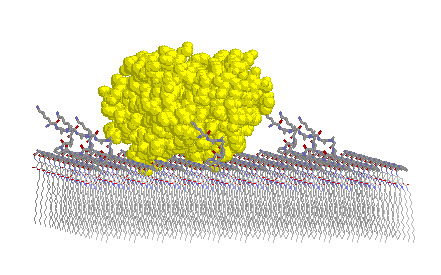
Figure 9 Top (left) and side (right) views of a monolayer lattice of peptidic lipids with a trypsin molecule in an attempted docking with a peptide headgroup of one of those lipids.
6. Synthesize poly (glu) Yamada lipopeptide
Polyglu-didodecylamide (H-(Glu)13-glu-didodecylamide), as reported by Yamada's group, was synthesized as an exercise and a test system for microstructure formation and characterization by optical and electron microscopy. The product of following the literature synthesis proved to be a relatively poorly defined system because of variations in the length of the poly-Glu chain.
We chose working models with defined peptide chain length; three lipopeptides reported by Shimizu et al. [5] HCl.Gly-Lys(e-Z)-Sar-Pro-Glu-(NH-C12)2, 2HBr.Gly-Lys-Sar-Pro-Glu-(NH-C12)2, and HCl.Pro-Pro-Pro-Glu-(NH-C12)2 were synthesized. Characterizations of the resulting microstructures are in progress. Amphiphiles with peptide head groups containing tryptic cleavage site was designed by making minor modifications of one of the Shimizu peptides reported to form tubules and helical ribbons. Ac-Lys-Ala-Sar-Pro-Glu-(NH-C12)2 was synthesized; microstructure formation experiments are in progress. To acquire amphiphiles with physical properties (i.e., Tm and CMC) useful in the study of release kinetics by dissolution and tryptic cleavage, varying hydrophobic chain lengths (namely, Glu-(NH-C12)2, Glu-(NH-C14)2, and Glu-(NH-C16)2 were prepared for coupling to peptide head groups and as potential components in mixed lipid tubule formation.
We suspect that proline at the first position of the head group on a glutamine lipid core may have a role in determining the type of microstructures that are formed. Therefore, HCl.Pro-Glu-(NH-C12)2 was synthesized and found that it forms crystalline microstructures as examined by optical microscopy (see below). Further characterizations are being pursued.
In preparation for tryptic cleavage kinetic studies of the anticipated tubular amphiphile, Ac-Lys-Ala-Sar-Pro-Glu-{NH-(CH2)x-CH3}2, Ac-Lys-Ala-Sar-Pro.HBr is being prepared to ascertain that the peptide is a substrate for trypsin, and to determines the kinetic rates via derivatization and RP-HPLC separation of the cleaved peptides.
7. Synthesize initial Kunitake lipopeptide
It was decided that the close similarities of the tubule-forming core of the Kunitake and Yamada lipid systems were such that it made more sense to begin with the system that immediately allowed addition of polypeptides (the Yamada or Shimizu lipid system). The essential difference between the lipids is the presence of ester linkages in this type of lipid. The amide linkages being used are chemically more stable and may be more likely to form inter-lipid hydrogen bonds.
8. Synthesize initial modified DC8,9PG
It was decided that the potential toxicity of the diacetylenic group made it less attractive than the other tubule-forming lipid systems, so synthesis on this lipid has been shelved for the moment. We will revisit it if there are unforeseen problems with any of the other systems.
9. Synthesize initial peptide-modified ceramides: galactocerebrosides (NFA) and aminoacyl-ceramides
Galactocerebrosides, known tubule formers, consist of a sphingosine base (1), fatty acid side chain and a galactose head group. To better understand the basis of tubule formation several chemical alterations of cerebrosides are being explored. In nature, galactocerebrosides exist as a nonhomogeneous species. The fatty acid side chain varies in length, degree of unsaturation and hydroxylation while the sphingosine base is either sixteen or eighteen carbons in length. A nervonoyl fatty acid side chain (2) and a sphingosine base comprising 18 carbons were employed to simplify the chemical synthesis and microstructure formation studies. In addition, nervonoyl (15%) and C18 sphingosine base (95%) account for a major portion of a given naturally derived galactocerebroside. Such a pure species, N-nervonoyl galactocerebroside (3) has been shown in this lab to form microstructures.

The first chemical modification involves the replacement of the sugar head group with amino acid(s). Commercially available mixed N-acyl ceramides (4) were hydrolyzed and the resultant sphingosine base acylated with the prepared N-hydroxysuccinimide ester of nervonic acid to provide intermediate (5). Next, the 1° alcohol was protected as the triphenylmethyl ether (6) and then the 2° alcohol was converted to the t-butyldiphenylsilyl ether (7). Treatment of this derivative with mild acid revealed the 1° alcohol (8) which was coupled to N-acetyl-proline using standard esterification methodology (9). Desilylation was effected by treatment with ammonium fluoride to provide the target molecule (10). Microtubule formation studies of this compound and its silylated precursor are currently under way.
The more difficult alteration envisioned consists of attaching amino acid(s) to the sugar moiety of the cerebroside. To achieve this the 2° alcohol of the triphenylmethyl ether ceramide derivative was benzoylated (11) and the 1° alcohol revealed upon treatment with mild acid (12). This ceramide derivative will be glycosylated by a protected galactose moiety (currently being synthesized) and then amino acylated.
10. Yamada lipopeptide: Optical microscopy, electron microscopy (TEM, ESEM, and FFEM), DSC, determine CMC with DCA, Raman spectroscopy
We performed differential scanning calorimetry (DSC) of lipids dispersed in buffer using our Seiko DSC-100 high sensitivity DSC. Samples were heated and cooled at 1°C/min. Phase transitions of some synthesized and commercially available lipids are summarized in Table I. Values obtained for DPPC, and DMPC are very close to those reported earlier [10] . To insure future accuracy we will calibrate the calorimeter monthly using double distilled water and DBPC (Tm 74°C).
|
|
|
|
DPPC |
|
|
DMPC |
|
|
Gly-Lys(e-Z)-Sar-Pro-Glu-(NH-C12)2, pH 5.5 |
|
|
Gly-Lys-Sar-Pro-Glu-(NH-C12)2, pH 5.5 |
|
HCl´Gly-Lys-Sar-Pro-Glu-(NH-C12)2, and (HBr)2xGly-Lys-Sar-Pro-Glu-(NH-C12)2 have phase transitions above room temperature. This is one of the important requirements to a tubule forming lipid, because all tubules observed so far were formed from lipids below Tm.
Several attempts were made to form tubules from the synthesized surfactants using precipitation from ethanol, or cooling of sonicated lipid dispersions through Tm followed by prolonged incubation. The resulting lipid microstructures were studied using a Zeiss ICM-405 inverted phase contrast microscope equipped with a DAGE 66 SIT video camera. Obtained images were converted into computer readable format using a Data Translation QuickCapture frame grabber board in a Macintosh II. TEM images of the microstructures were taken using facilities of the UW Medical School Imaging Center.
Glu-(NH-C14)2 and Glu-(NH-C16)2
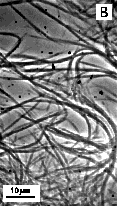
These lipids are particularly important, as they might be useful as the "small headgroup" lipids for use in mixed lipid system with drug-bearing surfactants with the same tubule-forming core, but with larger headgroups. Figure 10 shows optical images of particles obtained by precipitation of the surfactants from solutions in 75% ethanol upon cooling from 80°C to 20°C. TEM of the same samples (Figure 11) reveals long cylindrical substructures that appear similar to the cochleate cylinders found from HFA-cerebrosides. The appearance and diameter of structures on optical and electron images suggest that in 75% ethanol at room temperature, the elementary cochlear cylinder units (similar to that on Figure 11) aggregate, forming much bigger fiber-like structures. Elimination of ethanol from the system induces further aggregation, and formation of much thicker fibers (Figure 12). The tendency to aggregate can be explained by the fact that these surfactants have headgroups smaller than cross-section area of the hydrophobic region. Preliminary experiments suggest that mixtures of these surfactants with others that have identical bilayer-forming region, but bigger headgroup do form high-axial ratio microstructures. We will consider use of Glu-(NH-C14)2 and Glu-(NH-C16)2 as core lipids for presentation of peptidic lipids that have much bigger headgroups.
|
|
Figure 10.
Optical images of |
|
|

|

|
|
Figure 11. TEM image of Glu-(NH-C16)2 particle. |
Figure 12. Optical images of Glu-(NH-C16)2 particles in aqueous buffer. |
Figure 13. Optical image of Gly-Lys(e-Z)-Sar-Pro-Glu-(NH-C12)2 incubated at pH 6.35 for 5 days. |

|

|
|
|
Figure 14. TEM image of Gly-Lys(e-Z)-Sar-Pro-Glu-(NH-C12)2 particles obtained after 5 day incubation of sonicated dispersion of the surfactant at pH 7.6. |
Figure 15. TEM image of HCl.Pro-Glu-(NH-C12)2 particles
|
|
HCl´Gly-Lys(e-Z)-Sar-Pro-Glu-(NH-C12)2
We studied tubule formation ability of this peptidic lipid under conditions similar to those described above. Shimizu et al. reported that this lipid dispersed in water (pH 5.9-6.5) by sonication at 10°C above phase transition formed tubules upon incubation at room temperature "over a period of hours and days" [5] . Optical microscopy of our samples (Figure 13) did not support this assertion. Samples incubated at pH 6.36 formed large amorphous aggregates; decrease of pH to 7.65 induced crystallization of the surfactant. TEM images of the structures formed after 5 days of incubation are showed in Figure 14. It can be seen, that within the aggregates formed at lower pH, part of the surfactant is organized in tubule-like structures, but significant amounts of material remain in nontubular form.
Our results confirm that peptidic lipids do form tubules. They show, also, that passive incubation of surfactant dispersion is not a very effective tubule formation method. In the future we will study another approach such as precipitation from organic solvents, and slow cooling of the surfactant dissolved in organic solvent-water mixtures.
HCl´Pro-Glu-(NH-C12)2
TEM images of P-E(C12H25)2 particles formed by crystallization of this simple peptidic lipid are shown in Figure 15. The resulting particles vary greatly in diameter. Neither optical microscopy nor TEM allowed us to attribute the resulting particles to any certain class such as tubules, cochleate cylinders or crystals. In order to obtain more information about lipid organization in these and other lipid particles, we are in the process of resurrecting a derelict Balzers 360 Freeze Fracture instrument in the Center for Bioengineering. We expect this instrument to be functional within the month.
11. Kunitake lipopeptide: Optical microscopy, electron microscopy (TEM, ESEM, and FFEM), DSC, determine CMC with DCA, Raman spectroscopy
not pursued, as described above
12. Monitor dissolution/lysis kinetics of initial prodrug tubules in buffer using fluorescence monitoring
Studies on the polypeptide-based tubules are just beginning at this time.
13. Optical and electron microscopy of dissolution/lysis of prodrug tubules suspended in buffer
Studies on the polypeptide-based tubules are just beginning at this time.
We have performed a number of simulations of tubule hydrolysis as an aid in interpretation of the kinetic data that we have collected on the diacetylenic lipids, and on data that we will soon collect on other surfactant systems. We have been looking for characteristic features of the kinetics profile that can be explained by reasonable rules of behavior for interfacial catalysis. These models contain a minimum number of assumptions and variables. What follows is a description of one of the more elaborate and successful models we have developed to date. It reproduces some of the features we have observed by optical microscopy in the PLA2 tubule hydrolysis experiments.
Phospholipid tubule hydrolysis by PLA2 was simulated using a lattice model in which each lattice position had an occupancy of 0 or 1 corresponding to unhydrolyzed and hydrolyzed phospholipid. Applying periodic boundary conditions to only one of the lattice dimensions (e.g., the x) represents a closed cylinder, which mimics the simplest lipid tubule structure. The simulation uses a large pool of enzyme molecules that are either free in solution or bound to the tubule at any given time. Initially, all enzyme molecules are freely diffusing in solution. For each iteration, all enzymes are tested to see whether they (1) move to a new lattice position and (2) bind to the substrate (if they are free in solution).
The probability of a diffusion step depends on whether the enzyme is in solution (more mobile) or bound to the lattice (less mobile). Enzyme molecules can diffuse in all directions with equally likely probabilities. If the enzyme is bound to the lattice, each step changes the occupancy of the lipid in the new lattice position of 0 to 1 (e.g., each diffusion step automatically results in hydrolysis if the lipid in the new lattice position is unhydrolyzed). The probability for an enzyme free in solution to bind to the lattice depends on the occupancy number of the lipid in the lattice. The enzyme is 104 times more likely to bind to hydrolyzed lattice sites than unhydrolyzed lattice sites (to simulate PLA2's greater affinity to negatively charged/product interfaces). Binding only occurs if the lattice position IS NOT currently occupied by a bound enzyme. Binding to the lattice is irreversible.
Results, as shown in Figures 16-19, indicate that key features of the kinetics and the optical microscopic appearance of the hydrolyzing diacetylenic lipid tubules can be reproduced by this very simple model. We will see if aspects of this model can be directly tested in the next year.
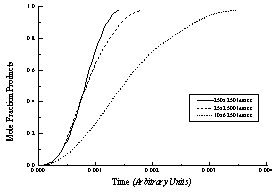
Figure 16 - Modeled reaction progress curves for three lattices with the same number of lipids (lattice sites) but different axial ratios (e.g., tubule length/circumference). All curves are "sigmoidal," but tubules with high axial ratios (long tubules) have lower overall hydrolysis rates and more linear hydrolysis profiles.
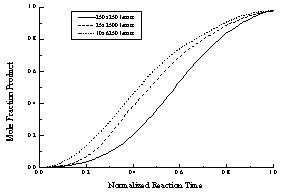
Figure 17 - The data presented in Figure A normalized to a constant hydrolysis time. The initial "lag phase," which is caused by the low probability of enzyme binding to a region of unhydrolyzed lipid, is more pronounced (relatively) for "square" lattices (small axial ratios).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0% |
|
|
20% |
|
|
40% |
|
|
magnification of left edge of 40% |
|
|
60% |
|
|
80% |
Note that this is only one of several possible models of interfacial hydrolysis. In future years we will construct competing models and compare the results of running these models with experimental data.
The project is operating on schedule. Work is more advanced in some areas, largely because of decisions not to pursue areas that do not for the moment seem competitive with the work being pursued. We have made a convincing demonstration that slow 0th order enzymatic hydrolysis of lipid tubules is possible, even in the face of irregularities in the structure of the lipid tubules used. This is a very promising first step along the way toward drug delivery, and a seminal study in the area of interfacial enzymology. We foresee both commercial interest in this work from the drug delivery community as well as great basic research interest in these novel enzymatic substrates.
1. N. Nakashima, S. Asakuma, J. M. Kim and T. Kunitake, Helical superstructures are formed from chiral ammonium bilayers. Chem. Lett., 1984. : p. 1709-1712.
2. N. Nakashima, S. Asakuma and T. Kunitake, Optical microscopic study of helical superstructures of chiral bilayer membranes. J. Amer. Chem. Soc., 1985. 107: p. 510-512.
3. K. Yamada, H. Ihara, T. Ide, T. Fukumoto and C. Hirayama, Formation of helical super structure from single-walled bilayers by amphiphiles with oligo-L-glutamic acid-head group. Chem. Lett., 1984. 10: p. 1713-1716.
4. H. Ihara, K. Yoshikai, M. Takafuji, C. Hirayama and K. Yamada, Amphiphiles with polypeptide-head groups. 7. Relationship between formation of helical bilayer membranes and chemical structures of dialkyl amphiphiles with polypeptide-head groups. Kobunshi Ronbunshu, 1991. 48(5): p. 327-34.
5. T. Shimizu and M. Hato, Self-assembling properties of synthetic peptidic lipids. Biochimica et Biophysica Acta, 1993. 1147: p. 50-58.
6. D. D. Archibald and S. Mann, Self-assembled microstructures from 1,2-ethanediol suspensions of pure and binary mixtures of neutral and acidic biological galactosylceramides. Chem. Phys. Lipids., 1994. 69(1): p. 51-64.
7. N. Nakashima, S. Asakuma, T. Kunitake and H. Hotaini, Dynamic transformation of the morphology of dialkylammonium bilayer aggregates. Chem. Lett., 1984. : p. 227-230.
8. P. Yager and P. E. Schoen, Formation of tubules by a polymerizable surfactant. Mol. Cryst. Liq. Cryst., 1984. 106: p. 371-381.
9. D. D. Archibald, Structural studies of high aspect-ratio self-assembled lipid microstructures with the use of microscopy and FT-NIR-Raman spectroscopy. 1990, University of Washington:
10. D. Marsh, CRC Handbook of Lipid Bilayers. 1 ed. 1990, Boca Raton, FL: CRC Press. 387.
|
Return to <Yager's Home Page or to Tubule Project Page> |
|