Architecture
Architecture of the cholera toxin (CT) AB5 heterohexamer which is very closely related to the heat labile enterotoxin (LT) from enterotoxigenic E. coli.
The cholera toxin family: Action and Inhibition
Architecture
Architecture of the cholera toxin (CT) AB5 heterohexamer which is very closely related to the heat labile enterotoxin (LT) from enterotoxigenic E. coli.

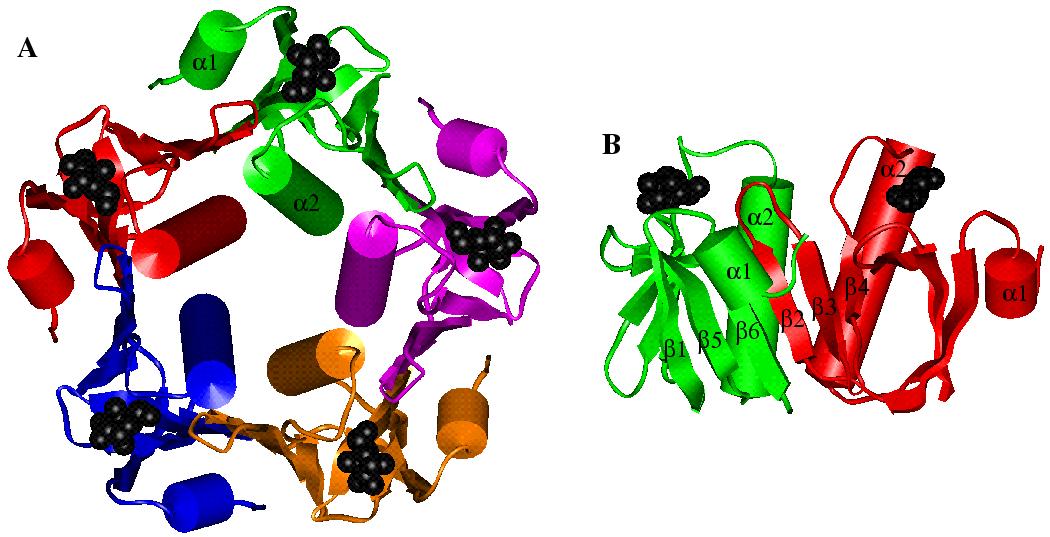
Schematic diagram of (a) the B pentamer of heat-labile enterotoxin, with each of the B subunits depicted in a different colour and the galactoses in each subunit depicted in black, and (b) two of the B subunits forming the six-stranded antiparallel b sheet.
Designed inhibitors in complex with the 5 receptor binding sites of the toxin
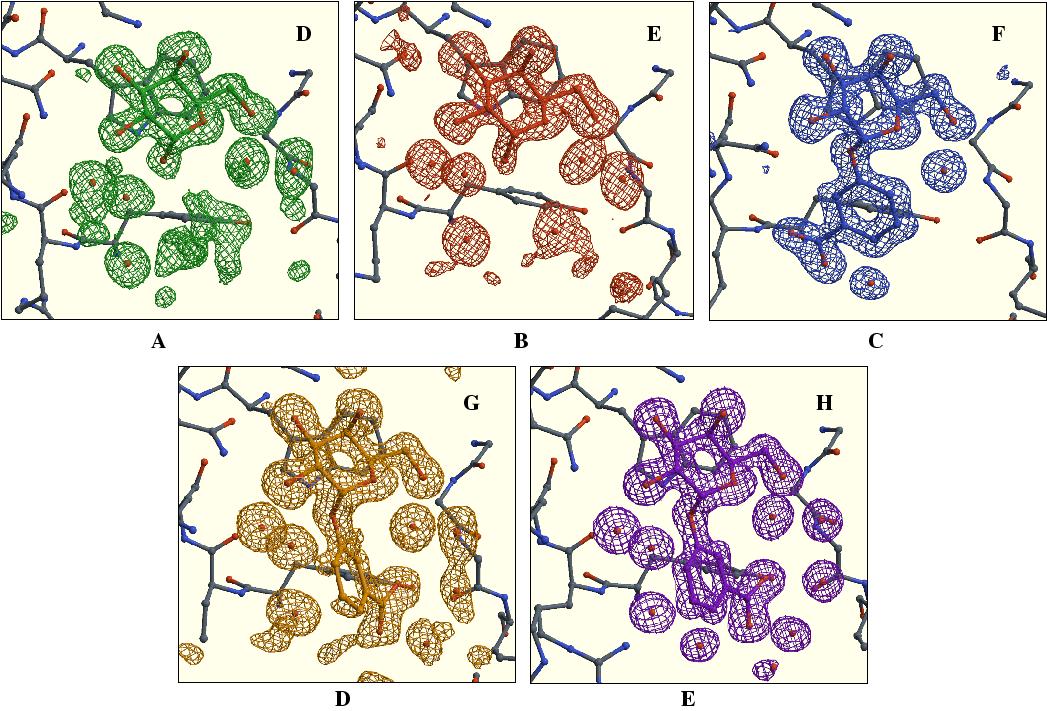
The five receptor-binding sites and their ligands in sA-weighted
(mFo - Fc) electron-density map in which the atoms
belonging to the ligands have been omitted along with some important waters.
(a), (b), (c), (d) and (e) show subunits D, E, F, G and H, respectively.
The contour level in (a), (b) and (d) is 3s, while
the contour level in (c) and (e) is 4s. In
each panel, the ligand as modelled in the corresponding binding site has the
same color as the electron density in that site.
Synergism of structure-based drug design with combinatorial chemistry for the design of receptor antagonists of cholera toxin:
Toward the structure-inspired design of cholera toxin assembly inhibition:
MDT: 3-methylthio-1,4-diphenyl-1H-1,3,4-triazolium
- a candidate ligand which might "plug the pore", thereby inhibiting cholera
and related AB5 toxins.
Electron density for MDTs in the co-crystal structure with porcine
heat-labile enterotoxin b pentamer.
For more information about this subject, please see the Research Summary Part II Section 3.