
(The drawings were created using RasMol ,the elegant freeware program for displaying PDB and other structural files. The animation was created using the scripting program Gifbuilder.)
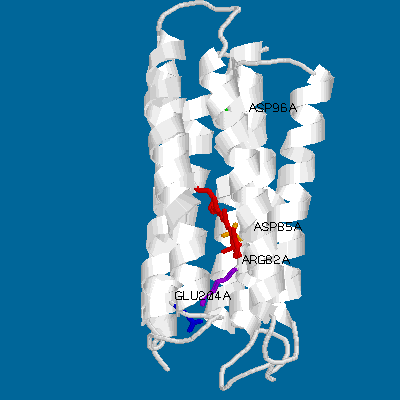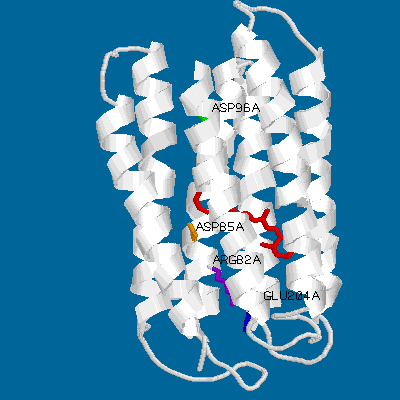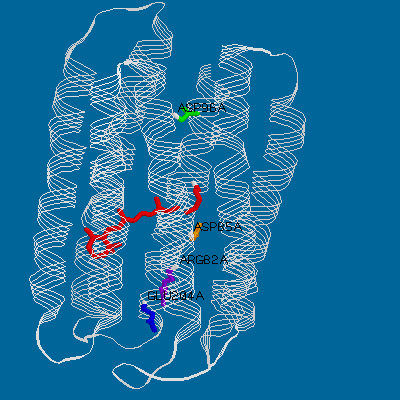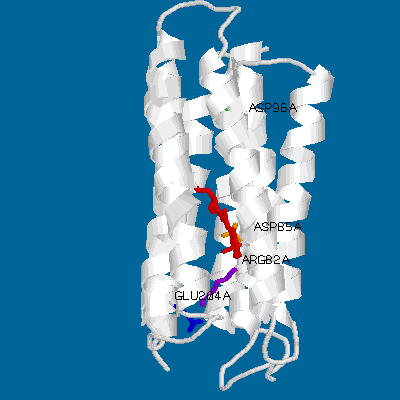
Even a simple picture like this one can display the main features of bacteriorhodopsin. Its main structural characteristics, the seven transmembrane helices, are represented as grey strands. The loops connnecting the helices are newly resolved in the refined Henderson structure but still have relatively high temperature factors compared to the helical domains. Several key groups are highlighted: The light-absorbing retinal chromophore (linked to Lys-216 via a protonated schiff base) is shown in red, and is in its ground-state (all-trans) configuration. Also shown are the important residues asp-85 (yellow), arg-82 (purple), glu-204 (blue) and asp-96 (green).
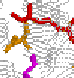 Click to download the pdb structure file (br.pdb; 178kb)
for bR. If you have RasMol
or a similar program set up as a helper on your browser, you should be able
to display the structure immediately. A view from "above" or "below" the structure
clearly shows the oblong arrangement of the seven helices, surrounding a channel
which is occupied by the retinal and lined with the residues mentioned above.
Click to download the pdb structure file (br.pdb; 178kb)
for bR. If you have RasMol
or a similar program set up as a helper on your browser, you should be able
to display the structure immediately. A view from "above" or "below" the structure
clearly shows the oblong arrangement of the seven helices, surrounding a channel
which is occupied by the retinal and lined with the residues mentioned above.
 Upon absorption of a light quantum (Amax at 568 nm), bR undergoes a photocycle
initiated by the all-trans to 13-cis isomerization of the
retinal, and characterized by a series of distinct spectral intermediates.
During the photocycle, a proton is transferred from the schiff base
to its counterion, asp-85. Concurrently, a proton is released on
the extracellular side of the membrane, most probably from glu-204
with the help of arg-82. This leads to the formation of the strongly
blue-shifted (Amax around 410 nm) M intermediate.
Upon absorption of a light quantum (Amax at 568 nm), bR undergoes a photocycle
initiated by the all-trans to 13-cis isomerization of the
retinal, and characterized by a series of distinct spectral intermediates.
During the photocycle, a proton is transferred from the schiff base
to its counterion, asp-85. Concurrently, a proton is released on
the extracellular side of the membrane, most probably from glu-204
with the help of arg-82. This leads to the formation of the strongly
blue-shifted (Amax around 410 nm) M intermediate.
Subsequently, in the N intermediate,
the schiff base is reprotonated from asp-96, which is itself
reprotonated from the cytoplasmic side of the membrane. In the O
intermediate, the retinal reisomerizes to an all-trans configuration,
while the proton release group glu-204 is reprotonated by asp-85.
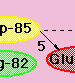
Click on the thumbail at left to see a schematic
representation of the proton pumping process (steps 1-5).
Not surprisingly, there are many pH-dependent processes, ostensibly
linked to the protonation states (pKa values) of different residues, which
modulate the function of bR. For example, protonation of the counterion
asp-85 in the ground state causes a red shift in the spectrum, called
the purple-to-blue transition.
 You can see this explicitly in this picture.
You can see this explicitly in this picture.
(click to enlarge)
Another example of pH-dependent behaviour in bR is the temporal reversal of proton uptake and release when the solution pH is lower than the pKa of the proton release group, Glu-204. In this case, the proton is thought to come directly from Asp-85, during the decay of the O intermediate. Examine the proton pumping scheme once more, looking at step 5' this time.....
We examine bR using steady-state and kinetic UV-Vis spectrophotometry,
as well as using a home-built photocurrent setup to measure charge movements
directly. We have been concentrating, as of late, on site-directed mutants
of bR prepared by collaborators in the departments of opthalmology (Dr.
R. Crouch) and cardiology (Dr. D. R. Menick), at the Medical
Univ. of S. Carolina. To find out more about bR, please take a look
at some pages discussing our recent research
![]() Back
to Ebreylab Home Page
Back
to Ebreylab Home Page