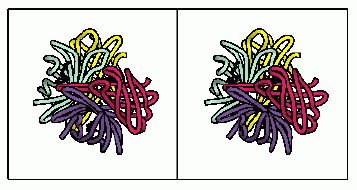
Streptavidin is a tetrameric protein which binds very tightly to the small molecule, biotin. The binding constant for this interaction is very high and has made the streptavidin/biotin system the focus of a number of studies aimed at determining what particular intermolecular interactions give rise to the tight binding. If this strong binding can be understood, it should help in probing other systems where similar interactions are important. In particular, the design of new drugs and ligands for proteins and nucleic acids will benefit from having a detailed understanding of the interactions involved. In collaboration with Pat Stayton , in Bioengineering, we are using a combination of mutagenesis techniques, thermodynamic measurements, and crystallography to systematically alter the amino acids involved in biotin binding to determine their effects on the binding. Stefanie Freitag and Isolde Le Trong are carrying out the crystallographic refinements and analyses of several complexes of streptavidin mutants and biotin analogs.

Above is a stereoview of the streptavidin tetramer showing the subunit arrangement and the biotin binding site.
Stefanie Freitag has also organized a streptavidin website.
Figures drawn using MOLSCRIPT (Kraulis, P.J., J. Appl. Cryst. 1991, 24, 946).
copyright © Ron Stenkamp stenkamp@u.washington.edu Most recent update 12/23/98
Back to Ron's homepage