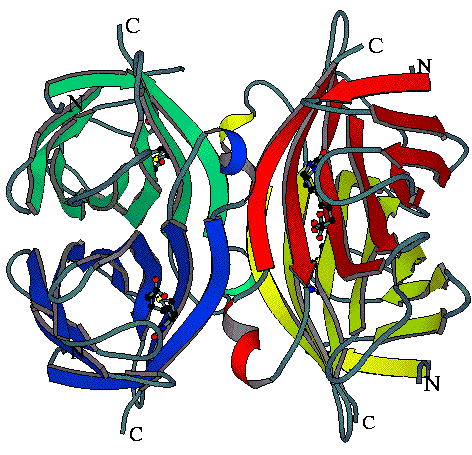
PI: Ron StenkampStaff: Isolde Le Trong
Postdoc: Stefanie Freitag
Cooperation with Pat Stayton's group, Department of Bioengineering (UW)
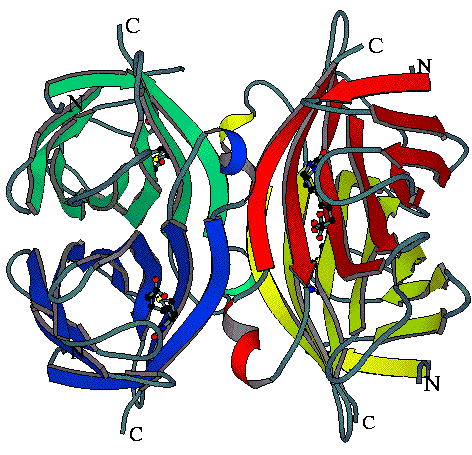
Streptavidin is a tetrameric protein (4 x 13kDa) that has various biochemical applications. The reason for this is the high affinity of the protein to biotin (Ka ~ 1013 M-1). Each monomer of streptavidin binds one molecule of biotin. The binding pocket is constructed showing three different binding motifs with the ligand as reported in the structures of the streptavidin-biotin complex by Weber et al., 1989 and Hendrickson et al., 1989 and also observed in our structures:
- hydrophobic and van der Waals interactions of mainly four streptavidin tryptophan side chains,
- an effective hydrogen bonding network,
- a binding surface loop, which folds over the ligand.
The major goal of our studies is to dissect the factors governing the binding in this high-affinity system. In collaboration with Pat Stayton we are using a combination of mutagenesis techniques, thermodynamic and kinetic measurements and crystallography to systematically alter the amino acids involved in biotin binding to determine their effects on the binding. The structures of several streptavidin mutants, with and without biotin bound, have been determined at resolutions of 2 Å. This includes mutants of the tryptophan and hydrogen bonding residues, as well as loop deletion mutants.
Understanding the structure-function relationship in this model-system for high affinity is relevant to systems where similar interactions are important. In particular, the design of new drugs and ligands for proteins and nucleic acids will benefit from having a detailed understanding of the interactions involved.
Publications:
S. Freitag, I. Le Trong, L. Klumb, P.S. Stayton, R.E. Stenkamp; Structural Studies of the Streptavidin Binding Loop; Protein Science 6 (1997), 1157 - 1166.
V. Chu, S. Freitag, I. Le Trong, R.E. Stenkamp, P.S. Stayton; Thermodynamic and Structural Consequences of Flexible Loop Deletion by Circular Permutation in the Streptavidin-Biotin System; Protein Science 7 (1998), 848 - 859.
S. Freitag, I. Le Trong, A. Chilkoti, L.A. Klumb, P.S. Stayton, R.E. Stenkamp; Structural Studies of Binding Site Tryptophan Mutants in the High-affinity Streptavidin-Biotin Complex; J. Mol. Biol. 279 (1998), 211 - 221.
copyright © Stefanie Freitag
Most recent update 6/26/03