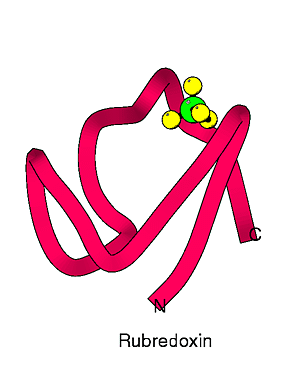
Rubredoxin is a small iron-sulfur protein found in various sulfur-metabolizing bacteria. Its biological function is not yet known for certain. Potential redox partners for the protein have been located in a few species, so it seems likely it serves as an electron transfer protein. The protein contains around 50 amino acids, and one iron is bound by the sulfur atoms of four cysteine residues. The iron-sulfur complex shows tetrahedral symmetry.
Rubredoxin from Clostridium pasteurianum was the first protein structure determined using X-ray crystallography at the University of Washington. In the late 1960's, Lyle Jensen and his group (Jon Herriott, Larry Sieker, and Keith Watenpaugh) solved the structure, and in the early 1970's, rubredoxin served as the test case for crystallographic refinement of proteins. In fact, rubredoxin was the first protein successfully refined. This led to a substantial increase in the computing activity associated with protein crystallographic studies and drastically improved the precision of the crystallographic models of macromolecules.
Rubredoxins from several sources have been characterized structurally, and all show the same fundamental polypeptide fold. The small size of the polypeptide and the constraints associated with binding a metal limit the amount of secondary structure. In one rubredoxin, that from Desulfovibrio desulfuricans, a deletion of several amino acids occurs in one loop lying on the surface of the protein. (Of course, with a protein this size, nearly everything is on the surface.) Presumably, this change in the surface could play some role in interactions with the protein's redox partners.
Studies are underway in several laboratories aimed at understanding how the amino acids in the protein affect the redox properties of the metal complex. Mutagenesis of the protein can affect the redox potential of the protein, but how that occurs is not yet known. A combination of structural and functional studies are needed to fully understand the principles governing redox behavior of this and other proteins.
See "Rubredoxin in the Crystalline State" by L.C. Sieker, R.E. Stenkamp and J. LeGall, Inorganic Microbial Sulfur Metabolism, Methods in Enzymology, Vol. 243 (1994).
Figures drawn using MOLSCRIPT (Kraulis, P.J., J. Appl. Cryst. 1991, 24, 946).
copyright © Ron Stenkamp stenkamp@u.washington.edu Most recent update 3/13/96
Back to Ron's homepage