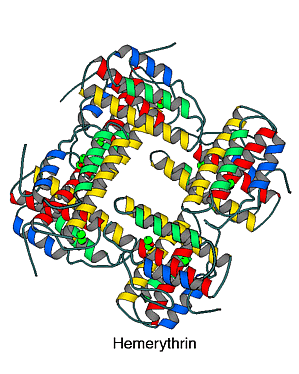
Hemerythrin is a non-heme iron protein used by two phyla of marine invertebrates (sipunculids and brachiopods) for oxygen transfer and/or storage. It differs from the other oxygen-binding proteins (hemoglobin and hemocyanin) both in the polypeptide chain and in the metal complex used to reversibly bind dioxygen.
The two iron atoms in hemerythrin are bound the imidazole rings of five histidine residues and the carboxylates of an aspartic acid and a glutamic acid. In addition, the complex contains an oxygen atom bridging between the two iron atoms. In deoxyhemerythrin, the bridge is a hydroxyl group, while in met- and oxyhemerythrin, the bridge is a mu-oxo atom. In deoxy- and metaquohemerythrin, one of the iron atoms is bound to six liganding atoms while the other is penta-coordinate. This extra site is where small molecules such as dioxygen or azide bind to the protein.
The fundamental polypeptide associated with each metal center is about 115 residues long. The protein is most commonly found as an octamer of molecular weight 108,000. In particular organisms though, dimeric-, trimeric- and tetrameric- forms of the protein are found. The folding topology of the polypeptide is that of a four-helical bundle. This motif has been found in a number of proteins. The wedged shape of such a bundle leads naturally to a binding pocket for the metal complex. Also, the dipole moments of the alpha helices are thought to aid in assembly of the octameric form of the protein.
In deoxyhemerythrin, the two iron atoms are in the ferrous oxidation state with a bridging hydroxyl group. As dioxygen is bound to the active site, the hydrogen atom from the hydroxyl bridge moves over onto the bound ligand, stabilizing the peroxo nature of the bound oxygen molecule. In met- derivatives of the protein, with and without small molecules bound to the complex, the iron atoms are both in the ferric oxidation state.
Most hemerythrin do not bind dioxygen cooperatively. They show tight oxygen binding, but the subunits in the oligomeric forms generally act as individual binding sites. In some brachiopods though, hemerythrin shows cooperativity between the subunits, so it will interesting to see what parts of the protein structure give rise to that inter-subunit communication.
For a more complete entry into the hemerythrin literature, see "Dioxygen and Hemerythrin", R.E. Stenkamp, Chemical Reviews, 1994, 94,715-726.
Figures drawn using MOLSCRIPT (Kraulis, P.J., J. Appl. Cryst. 1991, 24, 946).
copyright © Ron Stenkamp stenkamp@u.washington.edu Most recent update 3/13/96
Back to Ron's homepage