Transportation in the Post-Petroleum World
by
J. Richard Guadagno, Ph.D., P.E.
We have all heard people say that the world is running out of oil. We have also heard others claim that there is plenty left. But how can anyone determine for himself who is telling the truth? There is only one way, and it is not an easy one. To avoid simply depending on the opinions of others, a person has to possess one indispensable tool: a working knowledge of the principles of thermodynamics. This is the branch of physics which deals with energy and its interaction with matter, and it is largely defined by a set of basic laws. The First Law of thermodynamics may be familiar to many as either the law of conservation of energy or the law of conservation of matter. Both of these are combined in the First Law’s most basic form because in certain nuclear reactions matter can be transformed into its equivalent in energy, or vice versa. In this form, the First Law states that neither energy nor matter can either be created or destroyed.
The Second Law of thermodynamics is the basis of all cause-and-effect relationships in the physical world. It tells us that a transfer of energy – the cause – is necessary to bring about a physical change of any kind – the effect. Conversely, some kind of change must be expected from any transfer of energy. In its most basic form, the Second Law states that energy can spontaneously flow only from a higher energy state to a lower one. This is why – under normal conditions – water always runs downhill, heat always flows from a warmer body to a cooler one, and mountains always erode away. If you want to pump water uphill, turn a cool body into a warmer one, or move a fallen boulder from the base of a mountain back to its peak, you have to add energy from an outside source. And the amount of energy you must add is always at least as great as the difference in energy between the original and final conditions.
Why are these laws so overwhelmingly important? The answer is that the universe operates on an energy economy. Everything that happens anywhere in the entire universe depends on the availability of energy, from the behavior of a subatomic particle to the Big Bang, and from the tiniest biological process to the formation of a galaxy. If you have enough energy, you can accomplish anything. If you don’t have enough energy, you simply cannot do what you want. Over the centuries, millions of people have tried to do things which violate the First or Second Laws of thermodynamics – like turning lead into gold, or building a perpetual motion machine, or bending spoons by mind power alone. But no one has ever succeeded, and no one ever will. These two laws must always be obeyed; therefore it is they which must always be used as the ultimate arbiters of what is possible and what is not. And while other physical laws may tell us what happens, it is only the laws of thermodynamics which tell us why.
If you feel a bit overawed by these statements, don’t feel alone. Most people have never even heard of the scientific field of thermodynamics, let alone the First and Second Laws. I don’t know of any educational institution which grants a degree in the subject. Graduate engineers, physicists, chemists, and biologists get no more than a smattering of instruction about it. Only a few specialists, mainly in materials science or physical chemistry, systematically learn enough to allow them to apply its laws in an effective manner.
Ironically, astronomers and quantum physicists seem to be the most lacking in knowledge of this subject. This may be due to their inability to perform controlled experiments with their subjects, leaving them to depend solely on the mathematical expressions which describe them. Over the years I have discovered many elegant mathematical treatises, published in technical journals and even incorporated into textbooks, written by otherwise competent authors whose failure to apply the laws of thermodynamics has led them to produce nothing but glorious nonsense. As a result, our technical literature is full of misleading – and often embarrassing – misinformation.
We can’t afford to make this kind of silly mistake in predicting how long the age of petroleum is going to last. The First Law tells us immediately that petroleum, like all other natural resources, is limited in its abundance. But we must fall back on other devices to determine what these limitations are. After all, a number of times in the past people have said that we were running out of oil, only to be proven wrong when the discovery of new fields showed that our previous estimates of the amount available were in error. But things are different now, with this difference lying not only in a continuing increase in demand, but also in the methods used to discover new deposits. Until just a few decades ago, you simply drilled a hole somewhere and trusted in your luck. If you missed oil by only a few yards, you never knew it. But today we explore for oil by setting off seismic explosions and measuring the way the sound waves propagate through the subsurface geology. This not only lets us identify what kinds of strata lie beneath us and whether they are capable of containing oil, but it also lets us cover vast areas of land all at once. Most important, what today’s exploration methods tell us is not only where oil can be found, but also where it cannot.
By using these techniques, we have now succeeded in exploring virtually all of the land surface of the world, including the continental shelves. And we know full well that there are no major oil fields left out there still waiting to be discovered. By combining this knowledge with current trends in world petroleum consumption, a number of people – each of them working independently – have made calculations as to how much longer we can expect our oil resources to last. Not surprisingly, all of them have come up with almost exactly the same result: if we continue to follow the same methods of consuming our petroleum resources as we have in the past, the world will completely run out of oil by the year 2020. This date lies within two years of the one I calculated back in 1975, and it also agrees with recalculations I made only last year.
No one who has made such calculations has ever challenged either this conclusion or these figures. But there are plenty of others who have created highly imaginative reasons why they shouldn’t believe them. One person said "Oh, God would never let that happen!". But if God created the universe, mustn’t it also have been God who created the laws of thermodynamics? He’s not likely to repeal them just to protect one species from its own ignorance. Others claim that we don’t need oil because we don’t really have to travel; we can all stay home, doing our work electronically from there. To those we can say "fax me a loaf of bread". How could they not realize that when we run out of fuel for cars, we will also be out of fuel for trucks, buses, trains, tractors, airplanes, and ships as well?
Yet another common misconception of the effects of petroleum exhaustion is the failure to acknowledge that soon after we begin to run out of oil in a few isolated spots, we will be out of oil everywhere! This means that future transportation systems must be designed to serve every place where people reside – in the United States and in the entire world as well. Systems which serve only certain areas or certain segments of the population in any given area are – by themselves – inadequate, and can be of significant value only if they are designed to be components of an integrated whole.
The group whose denial of these facts is most dangerous is the world’s economists. Economics has long been defined as the study of the allocation of scarce resources. A far more realistic definition would be to replace the word "scarce" with "limited." I had been searching for a good way to demonstrate the difference between these two terms, and the 2000 election provided me with a perfect one. When asked what they were going to do about today’s high gasoline prices, Al Gore advocated releasing oil from our strategic reserves, while George Bush suggested that we put pressure on the OPEC nations to produce more. I’m sure that both of them consulted their economic advisors before making these statements, and it was no surprise that they could think of no solution other than to increase the supply. This would indeed ease the present scarcity, but in the long run it would badly deplete the limited supply. The only real result in either case would be that we might run out of oil in 2018 rather than 2020.
The word limited implies that the amount of petroleum – or any other natural resource which exists on the planet Earth – is a fixed quantity. In order to understand how this could be, one must understand the First Law. But this would contradict the basic concept underlying the economists’ own favorite invention, which they indiscriminately apply to all commodities: the law of supply and demand allows any resource to be acquired if one is willing to pay enough money for it. In following this tenet, economists really acknowledge only two quantities: plenty and none. By saying that there is still plenty of something left, they are simply saying that we haven’t run out yet. Unbeknownst to most economists, this "law" actually assumes that all natural resources are infinite in extent. This, of course, is a blatant violation of the First Law of thermodynamics.
The law of supply and demand does work fairly well for many resources, but what happens when we try to apply it to energy resources? To understand this, let’s see how it works for a typical resource: the metal copper. Man first started by using native copper – naturally formed nuggets of almost pure metal. All you had to do to use it was to pick it up and pound it into the shape you needed. But native copper could only be found in a few locations, and even there the supply was very limited. Soon it was all used up, and other materials – chemical compounds containing large amounts of copper – had to be employed instead. As time went on these higher grades of ore were also used up, and people continually had to revert to lower grades in order to obtain the copper they needed. In today’s world, we must literally process mountains of very low grade ore taken from gigantic open-pit mines in order to get the copper we need.
What is it that determines the grade of a copper ore? It isn’t really the concentration of metal. Instead, it is the amount of energy which has to be expended to recover a given amount of copper from the ore, and consequently the cost of that energy. This energy is needed for various procedures: to expose the ore, break it up, transport it to the mill, smelt it to separate the metal from the dross, and then chemically process it to remove impurities from the copper. This works fine for copper, and we will have some source of this metal for a long time to come, as long as we are willing – and able – to supply enough energy to get it out.
But simple common sense tells us that you can’t keep on throwing more and more energy at an energy resource and still expect it to produce more than you put into it. Sooner or later, the amount of energy needed to extract a fuel from the mineral matrix where it is found will grow to the point where it reaches the amount of energy which can be obtained from the fuel. When this happens, the supply can no longer be regarded as a net source of energy. And long before this point is reached, it becomes so low-grade that it is no longer practical to process it. Thus the law of supply and demand simply does not apply to energy resources. And it would be foolish for us to think that it does.
Unfortunately, most economists’ use of the law of supply and demand rather than the laws of thermodynamics causes them to act very foolishly in this regard. This is true both of free market economists, who base their laws on the availability of money, and Marxist economists, who base theirs on the availability of labor. But not all economists behave in this way. A few of them have been able to rise above what they have been taught and to see the world as it really is. E. F. Schumacher stated no later than 1964 that "energy is the pre-condition of all commodities." Nikolas Georgescu-Roegen proposed that, if we are to determine the true long-term value of non-renewable natural resources, we must employ the concept of entropy, which is a measure of the unavailability of energy, and which is derived from the Second Law. And Kenneth Boulding pointed out to us that anyone who believes that we can continue using these resources forever without eventually running out of them "is either a madman – or an economist".
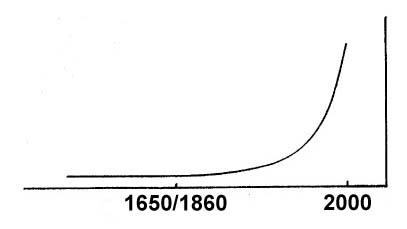
How did we get into our present predicament, where we will soon run out of the one resource – petroleum – which has become absolutely essential to the transportation systems which enable us to move about so freely and to have all the things we need brought to us? Let’s begin with a graph which is probably familiar to everyone.

Fig. 1. World Population as a Function of Time
This curve shows how world population has changed over time. For many centuries, world population remained at a very constant level of 500 to 600 million people. But then the industrial revolution let us utilize resources – and especially energy resources – far more effectively. It was that abundant power which allowed us to support an ever greater population. The increase continues unabated today, when 6 billion people inhabit our planet, ten times as many as were here before the year 1650.
At first, the industrial revolution was powered by coal. But when petroleum was first produced in quantity around 1860, it didn’t take long for it to become our most important source of energy, especially for transportation. Its rise has been so rapid, in fact, that the above population curve can readily be used to show oil consumption as well, simply by substituting the date 1860 for 1650. In fact, the oil curve is rising even more steeply than that of population, since per capita consumption also continues to increase. We are now burning it so fast that a year’s demand for oil today would have lasted decades not too long ago.
One thing this consumption curve tells us is that we must redefine upward the meaning of a new discovery of oil which is really significant. But how? I would like to suggest that we use the term "significant discovery" only for new fields which are able to extend the petroleum age by five years or more. That isn’t too unreasonable, is it? But when we look at the actual quantity that this implies, we find that the original oil reserves of the entire North American continent would fall short of meeting this definition. In fact, of all the original deposits in the world, there is only one which would qualify under this standard. This is the Persian Gulf field, which originally contained half of the world’s entire oil supply, and which now accounts for 3/4 of what is left. But if present consumption trends continued to the year 2020, consumption would be so high that even the discovery of something that big could prolong the oil age for only another eight years! And the First Law tells us quite conclusively that that there aren’t any more Persian Gulf fields out there waiting to be discovered. Thus we must accept the fact that the petroleum age is almost over, and that there is nothing we can do to prolong it. What we have to do instead is to learn to live in a world without petroleum. And we have to do this very quickly.
Since it is inevitable that we will soon run out of oil, what will our consumption curve look like in the future? The best estimates are that it will continue to rise at the same annual rate for about another decade. By that time, virtually all of the fields outside of the Persian Gulf will have dried up, and even its production capacity will begin to lag behind the demand. Consumption will fall off, and within a few years this drop will turn into a precipitous decline, as shown in Figure 2.
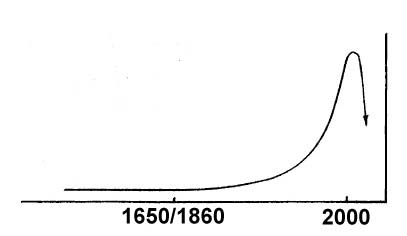
Fig. 2. Projected Future World Petroleum Consumption
What are we going to do then? I’m sure you have heard many people say that when we run out of oil, we will just have to start burning some other fuel in our cars’ engines. Natural gas has long been the favorite proposed alternative. Figure 3, which I prepared around 1990, shows the results of an analysis of the future of conventional domestic natural gas.
Fig. 3. Domestic Natural Gas Consumption
The flat curve came from the American Gas Association, the industrial organization of natural gas producers and distributors, and projected a 60-year supply – providing there was no increase in consumption. But this was an act of pure deception, designed only to increase sales and therefore profits. The AGA knew perfectly well that annual consumption was rising rapidly each year and would continue to rise in the future. My analysis incorporated the real consumption trends and followed the sloping curve instead. Current figures show that we are still following it quite closely today. It shows that, even without using natural gas as a motor fuel, our conventional domestic supplies are projected to run out at about the same time as global petroleum. Further development of Coalbed methane can extend this supply somewhat, but the really major deposit of this fuel, found in Colorado’s Piceance Basin, is plagued by the as yet unsolved – and perhaps unsolvable – problem of how to dispose of prodigious quantities of salt water which is up to four times as salty as the ocean, and which comes out of the wellhead along with the methane. Thus natural gas cannot possibly be a solution to our oil problem. In fact, we should acknowledge that it instead offers another problem that we must soon solve as well: instead of thinking of natural gas as an alternative motor fuel, we should really be thinking about alternative means of heating our homes after the year 2020.
How about other alternatives? We know that the world possesses large supplies of oil shale, tar sands, unrecovered heavy oils remaining in existing wells, and methane hydrate lying on the deep sea floor. All of these are mineral deposits which contain substantial quantities of hydrocarbons from which suitable motor fuels could be produced. In the thinking of some people, this fact automatically qualifies all of them as energy resources. Unfortunately, the thinking of these people does not include a grasp of the Second Law of thermodynamics. These resources are very different from the traditional energy sources coal, petroleum, and natural gas, which can simply be removed from the ground with a minimal expenditure of energy and then burned as fuels to heat and light our homes and propel our vehicles. The new ones must be considered instead as synthetic fuels, meaning that they must be artificially processed to separate the hydrocarbons from the mineral matrices in which they are locked up. These processes all demand the expenditure of energy from other sources. And the amounts of energy needed to do this are not insignificant.
As an illustration, scientific researchers in the field of controlled nuclear fusion have been trying for years to reach what they call the break-even point in their experiments. If and when they do it, it means that they will be able to produce as much energy as they consume. But they also recognize that they must go much further than this, and produce more than double the energy input, before the process can be considered feasible. This is illustrated in Figure 4.
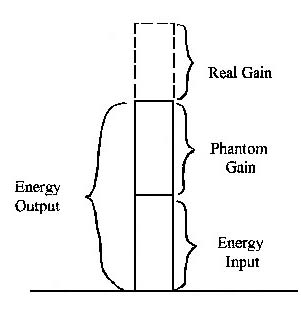
Figure 4. Energy Gains from Synthetic Fuels
If the amount of energy which can be derived from nuclear fusion is twice as great as the energy needed to produce it, then it appears that we have made progress. But this is really just a phantom gain. The increase at this point is equal to the input energy – which is no longer available – and we could just as easily have used that for the same purpose. Only the surplus amount lying above and beyond twice the input is a real net gain. This is not like winning a gambling bet, where you get your own money back plus a dividend. When you place an energy bet, it is gone forever!
This same situation holds for all potential synthetic motor fuels. Unfortunately, in this field the experiments have been conducted not by scientists, but rather by engineers from private corporations. My own personal experience with them has shown that, instead of treating the issue from a thermodynamic approach, these people tend to use antiquated trial-and-error methods instead, and they measure success only in monetary terms. Therefore they rarely even bother to measure energy input, and apparently don’t even realize that it has any importance.
All potential synfuels are chemically bonded to the mineral matrix they are found in. And the Second Law tells us that the long-term stability of this phase shows that this is the form in which the combination of hydrocarbons and minerals is at or near its lowest energy state. To get the fuel out, then, we must add an amount of energy from some other source which is greater than this bonding energy – in most cases a lot greater. This energy may be necessary for a number of reasons, depending on which synfuel we are dealing with, and it includes such diverse items as building roads, manufacturing equipment, digging up the ore, transporting it to a mill, breaking it up into small particles, thermally and chemically processing it, transporting the fuel, and disposing of any unwanted by-products.
Billions of dollars of taxpayers’ money have already been spent trying to find feasible ways of producing fuel from oil shale, tar sands, and heavy oils, without any positive results to date. But the failure to measure the real input and output energies of the process means that we don’t even know how far away the researchers were from devising a practical method of extracting the fuel. Of one thing we can be sure, however. The odds of any single one of these potential synfuels really proving practical are too low for us to rely on them for the entire future of our transportation systems – and the consequent survival of our civilization. We might be lucky, and one or another of them might actually provide a fuel which we can substitute for gasoline, but it will inevitably cost many times more than gasoline does today.
But the most frightening thing about this situation is that it doesn’t really matter much if we do succeed. We are living in a different world from that of a half-century ago. World population is now so great and per capita oil consumption so high that what was decades’ supply then now lasts only a year or so. To put it in a different perspective, if we were able to develop a new motor fuel today which produced a net energy supply equal to all of the world’s original petroleum deposits – an extremely unlikely possibility – it would allow us to continue the internal-combustion-engine age for less than 15 years. That doesn’t offer today’s young people a very long future to look forward to after four million years of evolution. We must aim higher, much higher. Our sole purpose should be to find a way to guarantee mankind a sustainable long-term future – and we certainly can’t do that with synfuels.
How about alcohol from biomass? There are those who claim that this is indeed a sustainable form of energy. But it has already been proven that we must burn more gasoline to grow, harvest, process, and distill corn than the amount we replace with ethanol. Our government’s continued subsidization of this counterproductive process has been "justified" by the claim that ethanol reduces air pollution. But it doesn’t; it just transfers a fraction of the air pollution from urban to rural areas. Therefore alcohol advocates are now turning to methanol produced by enzymatic conversion of fast-growing plants such as cottonwood trees or certain grasses. But this is such a hopelessly inefficient method of converting solar energy into fuel that – using the figures developed by the advocates of this process – we can readily calculate that the amount of land which would be needed to provide such a substitute for our petroleum fuels in 2020 would be equal to the area of all the 48 contiguous United States, plus Mexico! For it to succeed, therefore, we would all have to quit eating – or even living in houses.
There are also other major drawbacks to biomass energy. The National Renewable Energy Laboratory in Golden, Colorado claims that alcohol from biomass would not contribute to global warming. But when I tracked this claim down, I noticed that no scientific data were cited to back it up. The argument seems to be merely an assumption, and it appears to have been based on a simplistic and erroneous carbon cycle analysis – a method of using the First Law which tracks each atom of carbon from the carbon dioxide in the atmosphere to the growing plants, then through a controlled biological process into alcohol, and finally back to the atmosphere when the fuel is burned in engines. If NREL chemists had used the correct and complete carbon cycle, which follows a much longer path involving a long chain of living, air-breathing (and therefore CO2 producing) creatures of diverse sizes, they would have found that the use of methanol would contribute two to four times as much CO2 (and therefore global warming) as its energy equivalent of gasoline, with the higher figure representing the additional use of methanol to fuel the machines involved in the process. Even worse, the complete harvesting of crops, which is necessary for effective alcohol production from biomass, depletes the soil of its nutrients far more rapidly and more thoroughly than any existing agricultural practice. It can only be repeated a few times before the soil becomes so sterile that it is totally useless for growing any kind of crop.
Even hydrogen has been proposed as a substitute for petroleum fuels. Why not? It is a superb fuel which produces far more energy per pound than any other, and its only emission is clean water. There is only one drawback: it doesn’t exist! Even though hydrogen is the most common chemical element in the universe, it is not found in usable form anywhere on Earth. It must instead be produced artificially like synfuels, except that this process is already known to require the use of several times as much energy as that which we can get out of burning the hydrogen! Once again, the Second Law rears its ugly head.
To show that physicists and engineers do not have a monopoly on silliness, let’s look at a supposed breakthrough in producing hydrogen which was announced early in 2000. Biochemists from the University of California found that certain aquatic plants known collectively as pond scum could be made to emit hydrogen gas directly if they were placed in oxygen-free chambers and suitably processed. This discovery was heralded as a virtually infinite source of energy. This would be true if the world possessed an infinite amount of wetlands, but it doesn’t. Pond scum energy is just one more in the long list of processes which convert solar energy into some other form, just like petroleum, natural gas, coal, biomass, hydroelectric power, and wind. And like all the others, it is an extremely inefficient way of doing this. If the researchers had only applied the Second Law, they would have found that an acre of the most barren desert, covered with photovoltaic collectors, can produce more than a hundred times more hydrogen through the conventional process of water electrolysis than an equal area of the most fertile marshland.
Thus all of these supposed future sources of energy can easily be shown to be nothing more than fantasies which can’t possibly hope to solve our long-term energy problems. All of them, like petroleum, are minor by-products of solar energy, which is sent to our planet every day from a source which we know is going to last for at least another four billion years. And this form of energy is available in quantities hundreds of times greater than all of the others combined. Through the use of photovoltaic collectors it can be turned directly into useful electrical energy with an efficiency dozens of times higher than for any of the other processes. Wouldn’t it make far more sense for us simply to utilize solar energy in this way rather than to run it through some weird biochemical or meteorological process which wastes almost all of it? Actually, this process is not only logical; it is the only real choice that we have. When our descendants look back from a historical perspective on our present period of dependence on biological energy, including both contemporary and fossil fuels, they will see it as merely a temporary – perhaps even momentary – period of time. It just happens that we are currently living in the middle of that moment.
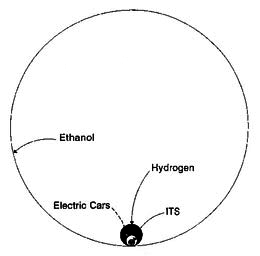
Figure 5. Efficiency of Solar Energy Utilization in Transportation
Figure 5, which comes from a report I prepared several years ago, demonstrates the relative feasibility of different methods of solar energy utilization. The area of each circle represents the amount of land which would have to be dedicated to energy production to power a specific type of future transportation system. The huge outer circle represents – with fair accuracy – the growing of crops to produce either ethanol or methanol, as well as hydrogen from pond scum. The others all rely instead on photovoltaics. The black circle is the amount of solar collector area needed to achieve the exact same result by generating hydrogen from water by electrolysis, and then using fuel cells to produce electricity from it. The smaller open circle is the area needed to use the same electricity to operate battery-powered electric cars. The tiny shaded one shows the area needed to transport the same vehicles by propelling them on the most advanced of a number of proposed light-rail systems which use an extremely efficient electrical propulsion device known as a linear synchronous motor.
Ratios between these areas show that it would take 1500 times as much land dedicated to collecting solar energy to propel a vehicle burning alcohol from biomass than to use the best rail system instead. Moreover, traveling on the rail system would be much cheaper, faster, and safer than driving cars powered by any chemical fuel. It would also be invulnerable to adverse weather conditions, would consume no non-renewable energy resources, would be entirely non-polluting, and would allow us to continue to enjoy all of the convenience and comfort of today’s automobiles. It would also halt global warming – but that is going to happen anyway when we run out of oil and gas, whether we survive or not. It would also grant to the United States and virtually all other nations of the world a goal which was first proposed during the Nixon administration, but which has since been abandoned as an impossible dream: energy independence. Most important of all, this is a truly sustainable system which will last as long as the sun shines.
Direct solar energy alone cannot solve all of our transportation problems. Combined with suitable energy storage facilities and transmission lines, it can easily be designed to power all ground transportation. This includes conventional railroads, all of which will eventually have to be electrified. While trains cannot be expected to compete with faster, cheaper, and more convenient light rail alternatives for passenger travel and most freight, they can still serve some purpose for shipping bulk goods such as coal (as long as it lasts), grain, and ore in unit trains, as well as a few items which are too heavy or too bulky to be carried by the light rail systems.
Both sea and air travel, however, must forever rely on other techniques. All ocean-going ships of the future are most likely to be powered by nuclear fission reactors. This is by far the most sensible use of this proven but still controversial energy source. First of all, our supplies of both uranium and thorium, the two raw materials for the production of fissionable materials, are quite limited, and should perhaps be preserved forever for this purpose. Secondly, the oceans are by far the safest place to use fission energy. The reactors can easily be designed both to survive the breakup or sinking of a ship, and to be readily retrievable if the ship is sunk in relatively shallow waters. If a sinking takes place in extremely deep water, then the reactor has already been disposed of in the safest place we can envision.
Airplanes are a different matter. In order to move through the air at reasonable speeds, they must be both light in weight and small in cross-section. They cannot carry a sufficient area of solar panels to provide the propulsion they need, nor can large amounts of electrical energy be transported to them through conductive wires. Therefore they appear doomed to eternal dependence on high-energy chemical fuels. In the future, these must be synthetic fuels, produced with solar-generated electricity from oil shale or similar sources. This means that air travel will inevitably be more expensive than it is today, but its cost should still be within a range reasonable enough for it to be used for rapid long-distance and intercontinental travel.
A combination of future rail lines and 300-mph magnetic levitation systems have been shown likely to produce faster door-to-door travel at lower cost than commercial airliners for distances up to about 2000 miles. Therefore airplanes would no longer be needed for travel within most of the continental United States. In the future, they are likely to remain as a viable transportation method only for coast-to-coast, intercontinental, and some inter-island travel.
It is obvious to anyone who is willing and able to apply the laws of thermodynamics to transportation problems that it would be insane for us to try to continue our present oil-burning systems for any longer than just a few years. It is also obvious that our chances of finding alternative fuels which would allow us to preserve the internal combustion engine for any significant period of time are negligible. This makes it equally obvious that three conditions must be met for any viable future ground transportation system. First of all, since we – like the rest of the universe – must operate on an energy economy, energy efficiency must be the most important consideration in selecting the ultimate system if we are to make the best use of the limited energy resources which are available to us. Secondly, the system must be electrically powered, since this is the only form of energy which can be transported over long distances easily enough to direct it to all the places where it will be needed. And third, the energy needed to produce that power must eventually be derived from the direct conversion of solar radiation. The longer we fail to acknowledge these facts, the less time we will have to make the conversion to a truly sustainable transportation system.
What if we don’t follow this path? What if we try instead to preserve the sacred internal combustion engine, betting everything on faith in economists and other shamans instead of the laws of thermodynamics? What will happen then? The answer is that if we don’t have an efficient, electrically-powered transportation system in place by the 2020s, we won’t have any effective transportation at all. And when we no longer have adequate transportation, we will no longer have civilization. To find out what this means, let’s go back one last time to Figure 2 and reinterpret it once again as a population curve. If we continue to follow our present course of action and ignore the lessons taught to us by the laws of thermodynamics – and the lessons of history – world population will inevitably follow the same catastrophic drop as oil consumption.
This is an incontrovertible fact. But it is a fact that very few people are willing to accept. Some have even suggested that we can all survive by going back to a "simpler" life. But we can see the impossibility of this course, and at the same time determine just how far world population might decline, by examining that mostly-ignored part of the population curve which preceded the industrial revolution. Why did world population remain so constant over such a long period of time – at least 1500 years and possibly much longer? It is now known that during this entire period, man occupied every portion of the globe which he could, including many areas which were more densely populated then than they are today. Thus it couldn’t possibly have been land still undiscovered which held the population down.
This leaves only one logical conclusion: the five to six hundred million population which prevailed during this long period of history must be the maximum carrying capacity of the planet Earth for a non-industrialized society. It would be logical to assume that the maximum carrying capacity of a world without an adequate transportation system would not be much different from this figure. This is no more than one-tenth of our present population. Take a good look at the next ten people you meet. Decide which of those ten you would choose to be the one to survive if we had to go back to a simpler life. Then, after you have done this, remember that each of these ten people may also be evaluating you.
But perhaps the most important thing to remember is that it doesn’t really have to be this way at all. There are ways in which we can solve our present and future transportation problems. But meandering into the realm of what is known to be thermodynamically impossible is not one of them.
Last modified: November 27, 2000