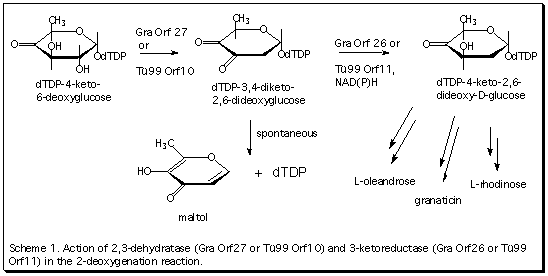
Antibiotics contain a variety of modified sugar moieties, mostly deoxy- and deoxyamino sugars, which are usually essential for biological activity. Most of these sugars lack an oxygen function at C-6, in addition, oxygens at C-2, C-3 and/or C-4 may also be missing. These deoxysugars are synthesized from D-glucose and the transformations take place at the stage of a dTDP sugar nucleotide. While the initial steps, catalyzed by dTDP-glucose synthase and dTDP-D-glucose 4,6-dehydratase are well understood, little was known about the subsequent deoxygenation reactions. Our group has recently cloned, sequenced and heterologously expressed genes involved in the biosynthesis of the sugar moieties of the antibiotics granaticin and oleandomycin. Two genes in each of these clusters encode enzymes which together catalyze the 2-deoxygenation reaction, i.e., the conversion of dTDP-4-keto-6-deoxy-D-glucose into dTDP-4-keto-2,6-dideoxy-D-glucose as shown in Scheme 1. Similar investigations are underway on enzymes presumed to catalyze 3-deoxygenation and 4-deoxygenation reactions. These studies make possible the enzymatic synthesis of a variety of dTDP derivatives of antibiotic sugars which can form the basis for a combinatorial enzymatic synthesis approach to novel "unnatural" antibiotic glycones.
