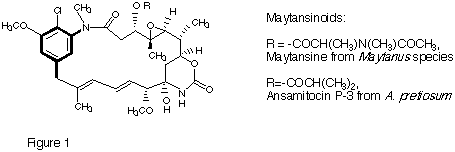

The maytansinoids (Figure 1), a group of ansa macrocyclic lactams with potent antitumor activity, were first isolated from Maytanus species and from members of 2 other plant families. They were subsequently also discovered to be metabolites of a soil bacterium, the Actinomycete Actinosynnema pretiosum. Maytansine itself was taken into clinical trials but, despite extraordinary activity in animals, turned out to be ineffective in humans, apparently due to dose-limiting toxicity. This makes the development of structural analogs a topic of considerable interest. Our group has embarked on studies of the molecular genetics of maytansinoid formation in A. pretsiosum with the aim of providing the tools for (a) genetically engineering the biosynthesis of new and structurally modified compounds in this family and (b) probing the relationship between microorganisms and higher plants in the formation of these unique compounds. These studies are a collaborative project with the group of Professor E. Leistner, University of Bonn, Germany.
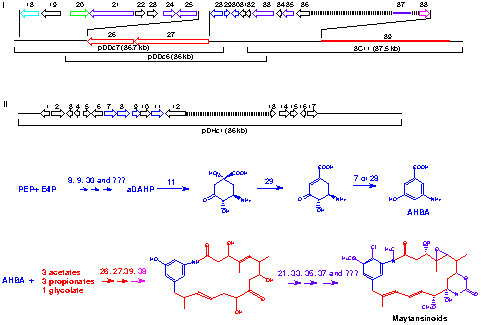
Using the AHBA synthase gene from the rifamycin biosynthetic pathway as a probe, we have isolated two clusters from A. pretiosum which contain AHBA synthase genes. The two clusters are located in proximity to each other (within 800 kb) on the A. pretiosum genome. Both clusters contain genes which are necessary for maytansinoid formation, but each is lacking some key genes expected to be required for the biosynthesis. It is possible that genes from both clusters are necessary for ansamitocin formation. These clusters are being sequenced (Figure 2) and evidence is being sought to confirm the assumption that they are involved in ansamitocin biosynthesis.