CrabLab, the research lab of
Katherine Graubard
Department of Zoology
University of Washington
Seattle WA 98195-1800 USA
![]()
![]()
Soc. Neurosci. Abstr. 20: 1414, 1994
TWO DISTINCT PATHWAYS FOR ACTIVATING cGMP IN THE CRAB STOMATOGASTRIC NERVOUS SYSTEM. N.L. Scholz, M.F. Goy*, J.W. Truman and K. Graubard. Univ. of Washington, Dept. of Zoology, Seattle, WA 98195 and *Dept. of Physiology, Univ. of North Carolina, Chapel Hill, NC 27599
. We are investigating pathways of cGMP activation in the crab stomatogastric nervous system (STNS), a collection of motor circuits known to be extensively modulated by both synaptically-delivered transmitters and circulating neurohormones. Using antisera selective for cGMP we have conducted parallel radioimmunoassay (RIA) and immunocytochemical (ICC) studies to screen for stimuli that activate cGMP synthesis in the STNS. We have found two classes of activators that are effective: nitric oxide (NO) donors and peptide-containing extracts of crab sinus gland.
In RIA studies, three NO donors (SNP, SIN-1, and SNAP) were all found to produce large increases in cGMP levels when applied with the phosphodiesterase inhibitor IBMX. A similar result was obtained with ICC studies: treatments with NO donors and IBMX consistently caused the appearance of cGMP immunoreactivity in a subset of neurons. We used an arginine-to-citrulline conversion assay to screen tissues in the vicinity of the STNS for a nitric oxide synthase (NOS), and found a candidate in crab heart. This tissue contains an arginine-metabolizing enzyme with properties that resemble a constitutive NOS, including calcium-dependence, NADPH-dependence, and sensitivity to arginine analogs. No such activity was observed in the STNS, the supraoesophageal ganglion, or muscle (GM1).
The sinus gland is a crustacean neurohemal organ that is known to contain peptides (members of the CHH/MIH family) that stimulate cGMP synthesis in a variety of target tissues. In RIA studies, extracts of crab sinus gland strongly elevated the cGMP content of the STNS when applied with IBMX. We are currently using cGMP ICC to identify which cells are targeted by the active component of the extract; preliminary results indicate that the peptide extract has a different target specificity than do the NO donors. Based on these results, we suggest that NO and a sinus gland peptide represent two non-overlapping pathways for activating cGMP in the STNS.
Supported by an NRSA Traineeship to N.S., NIH grants (NS 15697 to K.G. and NS 25915 to M.G.) and NSF grant IBN-9242993 to J.T.

MORPHOLOGY OF STOMATOGASTRIC NEURONS OF CANCER BOREALIS. K. Graubard*, A. E. Wilensky. Department of Zoology NJ-15, University of Washington, Seattle, WA 98195.
 The stomatogastric ganglion (STG) is a small pattern generator, comprised of 30
neurons.
For most neurons, including DG, LP, and PD, the primary neurite exits the ganglion
to become
a single large axon that does not divide until it nears its target muscles. However,
some neurons,
including IC, GM and VD, have axon divisions that occur close to or within the
ganglion. The
primary neurite of the IC neuron first branches to produce smaller neurites that
terminate within
the neuropil and then bifurcates near the edge of the neuropil into two
equal-diameter axons
which exit the neuropil without further branching. GM neurons have a similar
pattern; the
primary neurite branches repeatedly to produce 3-5 axons of unequal diameters, with
no further
neuropil branching. In contrast, the VD neuron's primary neurite splits early in its
course into
two equal-diameter neurites; they then branch extensively to produce smaller
neuropil-contained
neurites, after which the two large-diameter axons exit the neuropil. The regions of
neuropil
innervated by the VD's fine neurites are distinct, with little overlap. Since the VD has
two sites
of spike generation, it is interesting to speculate that this unique neuropil
organization subserves
some function. Supported by NIH grant NS15697.
The stomatogastric ganglion (STG) is a small pattern generator, comprised of 30
neurons.
For most neurons, including DG, LP, and PD, the primary neurite exits the ganglion
to become
a single large axon that does not divide until it nears its target muscles. However,
some neurons,
including IC, GM and VD, have axon divisions that occur close to or within the
ganglion. The
primary neurite of the IC neuron first branches to produce smaller neurites that
terminate within
the neuropil and then bifurcates near the edge of the neuropil into two
equal-diameter axons
which exit the neuropil without further branching. GM neurons have a similar
pattern; the
primary neurite branches repeatedly to produce 3-5 axons of unequal diameters, with
no further
neuropil branching. In contrast, the VD neuron's primary neurite splits early in its
course into
two equal-diameter neurites; they then branch extensively to produce smaller
neuropil-contained
neurites, after which the two large-diameter axons exit the neuropil. The regions of
neuropil
innervated by the VD's fine neurites are distinct, with little overlap. Since the VD has
two sites
of spike generation, it is interesting to speculate that this unique neuropil
organization subserves
some function. Supported by NIH grant NS15697.

CALCIUM CURRENTS IN STOMATOGASTRIC NEURONS OF CANCER PRODUCTUS. L.M. Hurley and K. Graubard. Department of Zoology, University of Washington, Seattle, WA 98195
The stomatogastric ganglion of decapod crustaceans is a major model system for studies of motor pattern generation. However, little is known about the properties of the calcium currents that are thought to underlie essential neuron behaviors. We are pharmacologically classifying calcium currents in 2 different stomatogastric motor neuron preparations: 1) in cultured somata and 2) at the GM neuron termination onto the gm1b muscle. Unidentified neuronal somata (50-100 um) were pulled from the ganglion by suction so that they had few or no neurites and were cultured ( after Panchin et. al. 1993) for 4-5 days. In order to reduce outward current and maximize inward current, TEACl (100 mM final concentration) and CaCl2 (26 mM final concentration) were substituted for equimolar amounts of NaCl and MgCl2, respectively. Up to 100 mM CsCl was also sometimes added to the bath. The intracellular electrodes were filled with 1M TEACl and 5mM BAPTA.
In two-electrode voltage clamp of cultured neurons, inward current (seen in 20/27 cells) showed rapid activation at steps positive to -30 mV and slow calcium-dependent inactivation. The current was not blocked by TTX ( 2uM, n = 4) and was blocked by the divalent cations Cd2+ (500 uM, n = 4) and Mn2+ (10 mM, n = 2). It was also reversibly blocked by the dihydropyridine nifedipine at concentrations of 50-100 uM (n = 9). In the absence of TEA, nifedipine and divalent cations reversibly blocked a component of outward current. At the GM-gm1b neuromuscular junction, nifedipine did not block evoked postsynaptic potentials (50-100 uM, n = 2). These results suggest that there are at least 2 calcium current subtypes in stomatogastric neurons.
Supported in part by NSF predoctoral fellowship and NIH training grant.

Soc. Neurosci. Abstr. 1995
CELLULAR DISTIBUTION OF NITRIC OXIDE-ACTIVATED cGMP IN THE DEVELOPING LOBSTER NERVOUS SYSTEM. N.L. Scholz, E.S. Chang+, J.W. Truman, and K. Graubard* University of Washington, Dept. of Zoology, Seattle, WA 98195 and +Bodega Marine Lab, Bodega Bay, CA 94923.
Nitric oxide (NO) serves as a signaling molecule in a diverse array of vertebrate
and invertebrate tissues. Nitric oxide activates a soluble guanylate cyclase, leading
to the production of cGMP. In the mammalian CNS, NO-activated cGMP is known
to mediate changes in synaptic strength; this signaling pathway may also play a role
in synapse maturation during nervous system development. We are studying
potential roles for NO and cGMP in identified circuits within the developing nervous
system of the lobster, Homarus americanus. In lobsters, postembryonic development
consists of three planktonic larval instars (stages I-III), followed by metamorphosis
and a transition to benthic life as a juvenile (stages IV & V). We stimulated the CNS
in vitro with exogenous applications of NO in combination with
isobutylmethylxanthine, and visualized NO-sensitive neurons with an antibody
against cGMP. Using this approach, we have mapped potential cellular targets for
NO at each stage of larval and early juvenile development.
The CNS of a stage I larvae contains hundreds of NO-responsive neurons. By
stage III, only a small subset of cells still respond; this sensitivity persists into
adulthood. For example, the segmental interneurons comprising the lateral giant
(LG) fiber system are sensitive to NO only during larval development, possibly
reflecting the establishment of synaptic connections that precede the emergence of
LG-mediated escape behavior. A unique pattern of NO-responsiveness is seen in the
stomatogastric ganglion (STG). Sensitivity first appears in a single neuron at stage
III. Additional neurons gradually become responsive during juvenile development,
until approximately 13 cells respond in the STG of adults. Based on these results, we
suggest that NO and cGMP may play an organizational role during development and
a modulatory role as a neuronal messenger in adult circuits. Supported by an NRSA
traineeship to N.S., NIH grant NS15697 to K.G, and NSF grant IBN9242993 to J.W.T.

PHARMACOLOGICALLY AND FUNCTIONALLY DISTINCT CALCIUM
CURRENTS OF STOMATOGASTRIC NEURONS. L.M.Hurley* and K. Graubard
Department of Zoology, University of Washington, Seattle, WA 98195.
We are classifying calcium currents pharmacologically in 3 different stomatogastric
motor neuron preparations in Cancer productus: 1) cultured somata, 2) neuromuscular
junction (NMJ) and 3) the intact ganglion.
In two-electrode voltage clamp of neuron somata cultured for 3-5 days, calcium
current is reversibly blocked by the dihydropyridines, nifedipine and nimodipine (50-100 M), but is not blocked by -Agatoxin IVa (300 nM). A component of outward
current is also blocked by nifedipine.
At stomatogastric NMJ, omega -Agatoxin IVa (300 nM) gradually blocks muscle post
synaptic potentials (psps) evoked by nerve stimulation. Nifedipine (50-100 M)
causes a brief burst of psps with each stimulus followed by an increased latency from
stimulus to psp and eventual all-or-nothing failure of psps. Nifedipine has little
effect on the amplitude of potentials evoked by pressure injecting acetylcholine
directly onto the muscle surface.
In the intact ganglion, these toxins have comparable effects, as measured by
extracellular nerve recordings and intracellular electrodes placed in cell somata.
omega-Agatoxin IVa applied to the stomatogastric ganglion produces a graded decrease in
the frequency of the pyloric motor rhythm and of the size of psps in the
stomatogastric motor neurons. Nifedipine causes a transient increase in the
frequency of the pyloric rhythm and in the number of units firing in nerves.
Nifedipine does not block psps, but does reduce the size of action potentials.
We suggest that there are at least 2 calcium current subtypes in stomatogastric
neurons, one important for spike-evoked transmitter release and the other for
activation of an outward current.
Supported in part by NSF predoctoral fellowship and NIH training grant.

Soc. Neurosci. Abstr. 1996
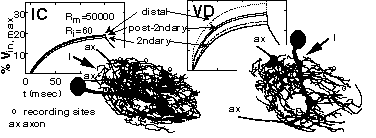
COMPUTATION STYLES IN IDENTIFIED NEURONS FROM THE CRAB STOMATOGASTRIC GANGLION. D.K. Hartline, K. Graubard, A.E. Wilensky and G. Orr. U. of Hawaii, Honolulu, HI 96822 and U. of Washington, Seattle, WA 98195
Intermixture of input and output synapses along neurites, as occurs in stomatogastric neurons, opens a possibility for "local" integration of inputs in relative isolation from other functional regions of a cell. The rate of attenuation of electrical signals spreading from a synaptic input to more distant neurites is a key factor in this. VD and IC neurons present very different morphologies. We compared signal attenuation in passive compartmental models of VD and IC to assess potential differences in computational capabilities. Serial optical sections of Lucifer yellow filled cells were made with a confocal microscope for 3D reconstruction with imaging software. For current injected in distal neurites of either cell type, attenuation of voltage responses was five-fold in secondary neurites and distal neurites "down stream" of them. In VD but not IC, attenuation was less in neurites having a secondary neurite in common with the injection site, and still less in those having a post-secondary neurite in common. The results suggest that local computation, while possible in both types, may be more regionally limited in IC than in VD cells (Human Frontier Science Program; NIH NS15697).
To be presented at the 26th annual meeting of the Society for Neuroscience in Washington D.C. November 17, 1996 8:00-11:30AM (Abstr. 59.5)

Soc. Neurosci. Abstr. 1996
SYNAPTOTAGMIN-LIKE IMMUNOREACTIVITY IN THE STOMATOGASTRIC NERVOUS SYSTEM OF THE CRAB, CANCER PRODUCTUS: A MARKER FOR SYNAPTIC NEUROPIL. J. M. Parrish, D. Goerlitz, K. Graubard. University of Washington, Seattle, WA. 98195.
Synaptotagmin is an abundant integral membrane protein found in synaptic vesicles, making it a good antigen for a synapse-specific antibody. The Drosophila synaptotagmin gene has been cloned and an antiserum (DSYT2) made to the highly conserved cytoplasmic domain (Littleton et al., Development 118: 1077-1088, 1993). Using confocal microscopy, we have found that the DSYT2 antibody (gift of Dr. Hugo Bellen) reveals synaptotagmin-like immunoreactivity in the stomatogastric nervous system (STNS) of the marine crab, Cancer productus.
The synaptotagmin-like staining on crab PD muscle fibers and in the STNS corresponds to known synaptic regions. Terminal varicosities on PD muscles stain similarly to those on crayfish muscle reported by Cooper et al. (Brain Res. 703: 214-216, 1995). In the STNS, synaptotagmin-like labelling is extensive and punctate. In the stomatogastric ganglion (STG) the label is intense and densely packed in the peripheral neuropil, while the core is almost devoid of staining. Neuropilar staining extends beyond the STG itself into the adjacent stn and dvn nerves. Patches of neuropil in the sons and at the stn-son junction also label. Some fibers in the stn and dvn stain near the STG and decrease in number as distance from the ganglion increases.
In preparations double-labeled for synaptotagmin and for the neuromodulator cholecystokinin (CCKC36), preliminary results show that anti-CCKC36 labeled profiles coincide with a subset of the total synaptotagmin-like labelling in the peripheral STG neuropil. Other preparations containing Lucifer Yellow-filled neurons were labeled with anti-synaptotagmin. Primary and secondary neurites occasionally traverse labeled regions. Fine neurites, however, nearly always lie in regions of synaptotagmin-like staining, often at the very edge of the neuropil border. Fine neurites that trail into the dvn and stn also lie in anti-synaptotagmin labeled regions. Supported by NIH grant NS15697 (to KG).
revised August 1996 whc