Home Michael Gelb News Research People Publication Positions Graduate Education
Home Michael Gelb News Research People Publication Positions Graduate Education
Research in the Gelb Laboratory
Major
accomplishments:
1. Discovery
of several human phospholipases A2 and determined their mode of action
and role in pro-inflammatory eicosanoid biosynthesis.
2. Discovery
of protein prenylation (farnesylation and
geranylgeranylation) of signal transduction proteins.
3. Development
of the first quantitative proteomic reagents (ICAT reagents).
4. Development
of several clinical candidates for drugs to treat tropical parasitic
infections.
5. Development
of most of the newborn screening assays added to screening panels over the past
10 years.
Current focus:
Newborn screening for treatable diseases in neonates
Newborns are sometimes born with a genetic disease that can be best treated if detected in the first few days of life (before irreversible symptoms develop). In the USA, almost all newborns (~4 million per year) undergo newborn screening. Most of the new assays added to newborn screening panels over the past 12 years were first developed in the Gelb lab. Research in this area is the lab's major focus. The work involves a mixture of synthetic organic chemistry and analytical chemistry mostly with tandem mass spectrometry.
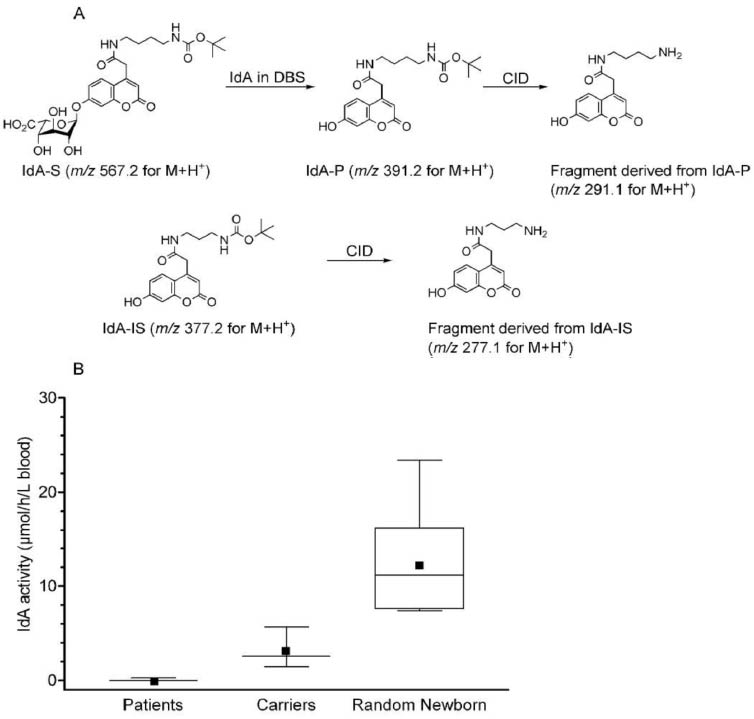
One example is shown below. The synthetic substrate is acting on by a glycohydrolase present in a dried blood spot on a newborn screening card (taking in the birthing center). The product is detected by tandem mass spectrometry in the presence of a chemically-identical isotopic-substituted enzymatic product allowing accurate quantification of the enzymatic activity. Newborns with a disease called MPS-I display enzymatic activity well below those without the disease. These test-positive newborns are then referred to a diagnostic lab, and treatment is initiated as soon as possible when warranted. Watch this Video to see how remarkable these treatments can be if caught early by newborn screening.
Drug discovery for neglected tropical diseases.
Chagas disease is a devastating diseased cause by infection with the parasite Trypanosoma cruzi. Chagas kills close to 1 million people annualy in S. America. Additionally, ~250,000 people living in the USA are infected with T. cruzi. There are currently no effective treatments for Chagas disease. The disease last for several years eventually leading to massive organ failure and death.
The Gelb lab carries out medicinal chemistry of anti-Chagas drug leads. The lab has been working for 2 decades with the Fred Buckner lab (Univ of Washington, Dept. of Medicine) on drugs for neglected tropical diseases. Our current project is focused on a new series of drug candidates that are the only agents known worldwide that can irradicate Chagas disease in mice as a mono-therapy. The program now involves a 3-way collaboration with University of Washington, the Drug Discovery Institute (University of Dundee, UK) and Glaxo Smith/Kline (Madrid, Spain). The hope in the next 1-2 years is to select a candidate for clinical trials.