Welcome to Comparative Biomechanics at Friday Harbor Laboratories
In this lab we apply principles from engineering and physics to living systems to better understand mechanics, materials, and structure in an evolutionary context. We often use marine fishes as investigative tools, but reptiles, amphibians, and even the occasional invertebrate make an appearance in the lab.
The Principal Investigator
My name is Adam Summers and I'm a professor at the University of Washington’s Friday Harbor Laboratories appointed in the department of Biology and in the School of Aquatic and Fisheries Sciences. For more on my background, check my biography.
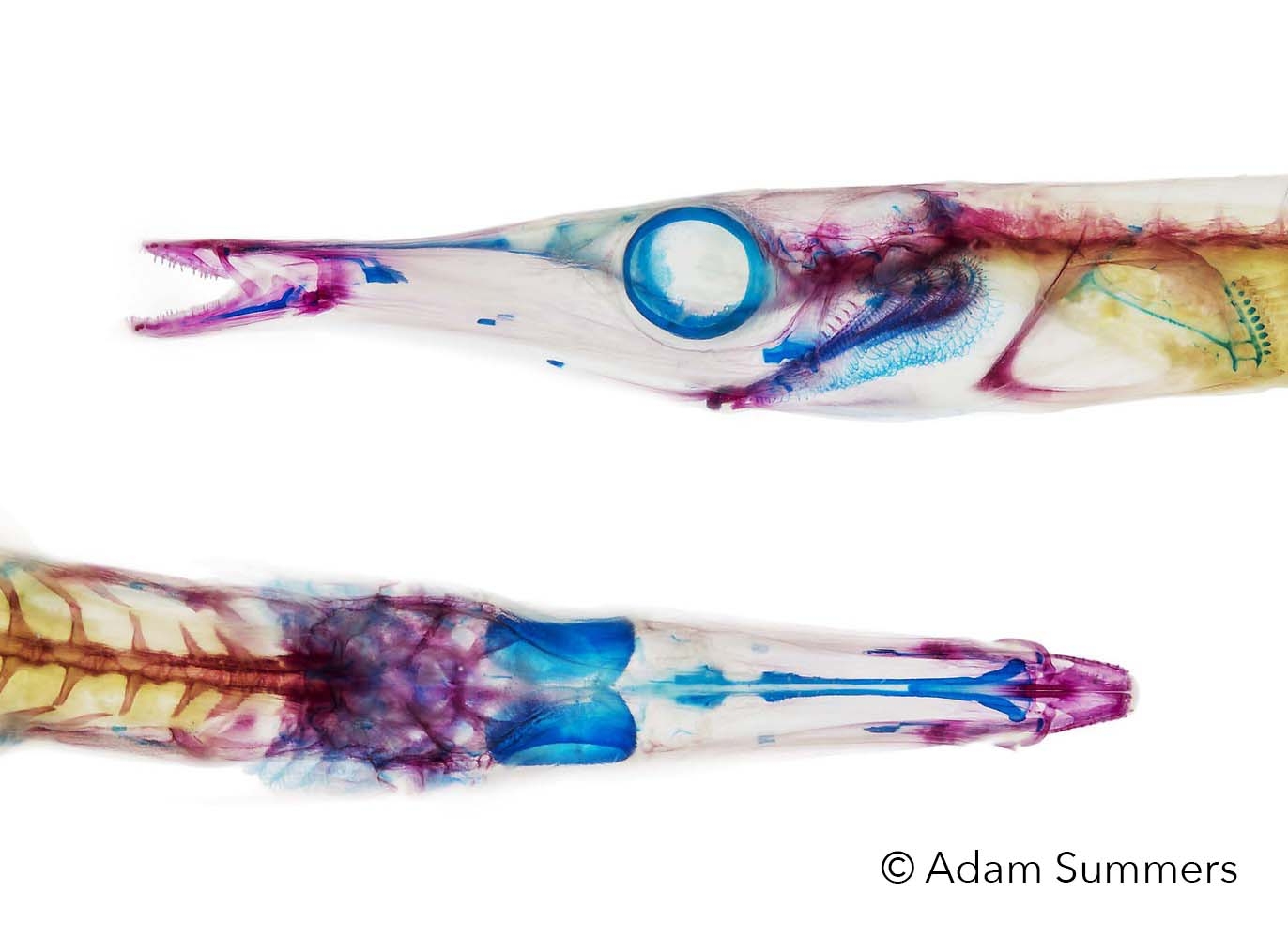
My research is heavily collections based and I hold research associate positions at the Burke Museum of Natural History, the Museum of Vertebrate Zoology at UC Berkeley and the Los Angeles County Museum. Current research topics include:
How fishes stick to things.
How fishes get under the substrate.
Tradeoffs in armor systems in fishes.
Measuring the performance of sharp and blunt teeth.
Filtering tiny particles from the water.
Free open source software for 3D visualization
@fishguy.bsky.social on bluesky
People
Learn more about the people in our lab, including current students and alumni.
Research
Click here to see what we've been working on recently and current venues of research.
#scanallfish
Read more about my project to scan all ray-finned fishes using CT technology.
To contact our lab, please direct packages and mail to:
Adam Summers
Friday Harbor Laboratories
University of Washington
620 University Dr.
Friday Harbor, WA 98250
email: fishguy (at) uw.edu
My office and lab are in Lab 8.
I do not have an office on the main University of Washington campus.
My post-docs are generally found around the labs and my graduate students split their time between main campus and the labs. Contact them directly by email to find out where they are.