What's simulation?
Simulation here is just one easy way to understand what is actually going on inside the microreactor. We build mathematical models which describe the reactor as best as we can. But on the other side, when models are too complicated, it might not possible to be solved even by the best computer. So we also need to make some assumptions.
About Matlab
Matlab is very powerful software, which has been used for quite a while for engineering or science applications. By using Matlab, we can actually solve our problem quite easily and present the results in a nice figure. By the way, the figures on this page are done in Matlab too, isn't it a pretty cool math software then?
Before simulation, you have to know...
Before you check out the simulation, you have to understand what things are involved in the simulation. First, you should know that there are 3 reactions as follows:
Reaction 1: ammonia + oxygen ® nitric oxide + water
4NH3 + 5O2 ® 4NO + 6H2O + 904 kJ
Reaction 2: ammonia + nitric oxide ® nitrogen + water
4NH3 + 6NO ® 5N2 + 6H2O + 1816 kJ
Reaction 3: ammonia ® nitrogen + hydrogen
2NH3 ® N2 + 3H2 – 94 kJ
By the way, the number 904 kJ, 1816 kJ and –94 kJ are actually the heat released by the reaction, where kJ stands for kilo Joule, which is a standard unit for heat energy. If the numbers are positive, it means that the reaction releases heat. Since our reactions have positive and BIG numbers, then it indicates that our reactions release a lot of heat. That’s one reason we want to have this reaction in a tiny reactor, so that we can control the heat to prevent it becoming too hot to actually blow the plant.
What have we known from Matlab simulation?
Can we neglect side reactions?
Now back to the reactions, we would be very happy if Reaction 2 and 3 do actually not have significant roles in the nitric oxide production. Because if it is true, we will not have to worry that Reaction 2 will consume all of our nitric oxide. Thus, The big question here is whether we can neglect those 2 reactions? The answer is NO.
The side reactions can not be neglected because it plays a big role in determining the final product of ammonia. For those who really want to know why, you can check out Can we neglect side reactions?
Second, you should know that there are temperature and pressure effects on the reaction. There are heat transfer and momentum transfers inside the microreactor as well. These things must be considered when we do the simulations, because we want our simulation to be close to the actual thing.
How does the temperature affect the reaction?
If we increase the temperature, the reaction should go faster, because molecules will bang to each other at much faster rate when it’s hot. Thus, we have more interactions between molecules, which increases the reaction rate.
How does the temperature change affect the reaction?
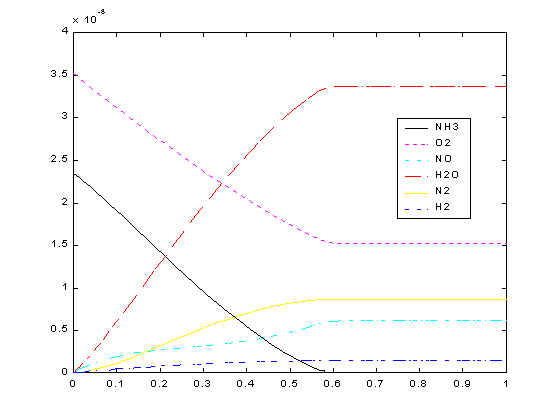
Now you know that the reactions release heat. What will happen is because of the heat released, temperatures inside the reactor become hotter and hotter as we go along the reactor. This increasing temperature consequently will increase the reaction rate. So the reactions are going faster and faster. However, we do not know whether we are going to get more or less nitric oxide with faster rate? That’s why we do simulations, whose results are shown below.

From the plot, you can see that ammonia and oxygen keeps going down as they move up the reactor. This is because none of the reactions produce ammonia or oxygen. On the other hand, nitric oxide goes up as it moves along the reactor. However, the rate of increase in nitric oxide slows down at some point. Do you know why? It’s because Reaction 2 starts consuming the nitric oxide produced in Reaction 1. Now you can actually prove from the simulation that the side reactions are significant.