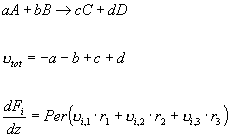
Few reactions in the gas phase maintain a
constant flow rate along the entire length of the reactor. To
determine the magnitude of the
change in flow rate we must sum up all of the stoichiometric
coefficients, as shown below.
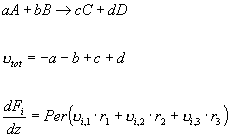
Where Per is the perimeter of the channel, ni,1 is the stoichiometry of the species, and r1 is the reaction rate. The total molar flow rate increases or decreases depending upon whether the summation is positive or negative, respectively.
In this case, the summation of the
stoichiometry turns out to be positive; the summation of the
entire series of reaction equals 1.5. Therefore, there are one
and a half more moles leaving the reactor than entering the
reactor per mole NH3 fed, assuming that the reactants
are feed in stoichiometric amounts
Want to find out more? Click on the General Model 1 page.