We are developing a treatment for paralysis whereby neural signals recorded from intact areas of the cortex are routed around an injury via an electronic device. This device can then send stimulation directly to nerves, muscles or the spinal cord below the injury in order to restore volitional control of movement.
We have thus far demonstrated that even neurons unrelated to movement can be used to control stimulation of muscles, and restore simple movements to an otherwise paralyzed wrist. This finding dramatically expands the potential control signals available for brain-computer interfaces and neuroprosthetic approaches.
Synchronizing stimulation can be used to strengthen connections among neurons via mechanisms of Hebbian plasticity. We are investigating whether intraspinal stimulation, triggered by appropriate cortical activity, is capable of guiding spared pathways to functional targets after spinal cord injury.
In collaboration with Phil Horner (Neurological Surgery), we are also exploring the possibility that stimulation may guide implanted stem cells to appropriate targets. Adult human fibroblasts, induced to pluripotency, will be directed to an astrocytic fate before implantation. Synchronous stimulation will then provide appropriate activity to strengthen functional connections across an injury site. We are hopeful that this combined therapy will create both a highly plastic environment and provide the necessary neural activity to repair damaged tissue.
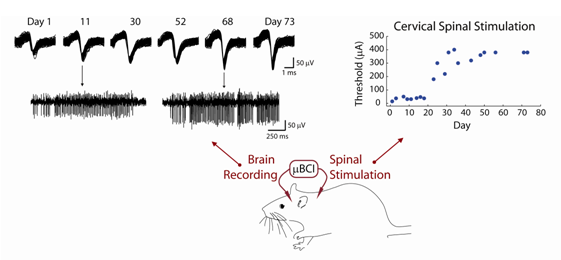
Cortical activity is used to trigger
spinal stimulation. Single
neuron action potentials from the same recording site are stable over many days despite some change in waveform amplitude (left). This brain activity will be used to trigger intraspinal stimulation to evoke forelimb movements using a microBCI (bottom).
Threshold currents for evoking movements via spinal stimulation
range from 20-500uA, and are stable over several weeks between occasional increases (right). From Otis, Moritz et al (IEEE EMBS in press)
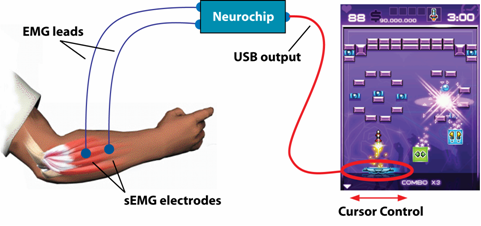
Children with spastic, paretic cerebral palsy (CP) often have weak or poorly coordinated muscles in one arm and hand. In collaboration with Sarah Westcott-McCoy (Rehabilitation Medicine) and Brian Otis (Electrical Engineering), we are investigating a novel treatment for CP where children receive augmented visual feedback of the activity of impaired muscles via a computer game interface. Thus children must contract and relax target muscles in coordinated patterns to control popular computer games (provided by PopCap games).