An Imaging Flow-Through Particle Analyzer
Abstract
With support from the Department of Energy we are completing the construction of a flow-through fiber analyzer for on-line monitoring and control of pulping operations. This unique instrument provides morphological and chemical information on individual wood fibers at high rates of speed. Central to this instrument is a novel sheath flow cell that hydrodynamically focuses the fibers into the focal plane of a fluorescence epi-illumination macroscope. Flash illumination freezes the particles’ motion, and the resultant fluorescence images are captured by gated CCD cameras.
Funding from CPAC is requested to explore applications of this technology to the on-line particle analysis needs of our sponsors.
Background
There is a need for automated analysis of particles in process streams. Detailed morphological and compositional information can be obtained using traditional microscopic techniques with manual inspection by highly trained personnel. A drawback of this approach is that is tedious and time-consuming, and therefore has limited application to process control. Analysis systems are required that can automatically quantify properties of individual particles, such as length, shape, and chemical nature, and produce a report that aids the optimization and control of the process. Fortunately, machine vision hardware and software are now sufficiently advanced for automated image analysis to be a viable alternative to manual inspection. However, conventional image processing instruments still require the samples to be mounted on a slide and manually loaded into the instrument.
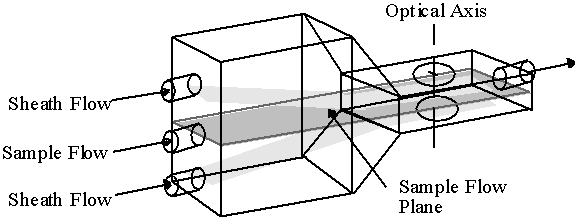
We have recently developed a particle image analyzer which presents the objects to the instrument by means of a continuously flowing sample stream. The unique hydrodynamic optical cuvette of this instrument uses two ensheathing aqueous streams that hydrodynamically focus the sample stream into a flowing ribbon of particles so that all are in optical focus. Figure 1 shows the operation of the flow cell. Not only do the ensheathing flows focus the sample stream, but they also prevent contact of the sample with the optical window, thereby minimizing fouling. Flash excitation provides an effective means of stopping the motion of the fast moving particles without blurring. By coupling a pulsed light source with a CCD camera, high quality digital images can be easily obtained. Now that high speed processing is available for reasonable cost, the resultant images, containing multiple particles, can be analyzed for both morphological and chemical properties with the full power of image analysis. Thus this instrument is intermediate between flow cytometers which analyze particles at high rates of speed, but provide limited morphological information, and static slide-based imagers, which provide detailed morphological analysis but at low rates of speed and without the advantage of automated presentation to the analyzer.
The pulp and paper industry is one industry that can benefit from automated suspended particle analysis. In paper manufacturing, the pulping stage digests wood chips into individual fibers. These fibers are eventually reformed into paper. The pulp fiber properties determine the quality of the final paper product, and as such the fiber properties need to be measured on-line in order to optimize the digester conditions. The Pulp and Paper Research Institute of Canada has developed a Fiber Quality Analyzer (FQA), which uses the flow cell in Figure 1 to image fibers entrained in the sample stream (1). The FQA measures the length of individual fibers, as well as their straightness and whether they are kinked. These properties all influence the mechanical strength of the resulting paper.
Kappa number is an index used by the pulp and paper industry to express the lignin content of a pulp. Lignin is responsible for the brown coloration of paper, and is removed by bleaching. Bleaching, however, also degrades the mechanical strength of the fiber. Therefore, the lignin content must be well known, so that only a minimum amount of bleach is used. The standard kappa number test consists of a redox titration that measures the bulk lignin content of a pulp sample.
It is known that there is a wide distribution of lignin content within commercial pulp digesters. There are many sources of this variation and many length scales over which the variation occurs. Differences in liquor circulation or in the temperature of different areas of the digester cause variation based on the location of pulp in the digester. Variation of this type can be quantified with the standard kappa number test because the sample size required for the test is small enough to resolve low frequency variation within the digester. However, there is also variation on a smaller scale that cannot be seen with the standard kappa number test. Sources of this type of variation include uneven penetration of chemicals into the wood chip due to mass transfer limitations, as well as differences in the fibers themselves, such as what time of year they were formed and what part of the tree they came from. To resolve this latter source of variation, a single fiber kappa number test is necessary.
Studies by one of the PIs (Gustafson) have shown that variation of kappa number from fiber to fiber may have significant impact on pulp bleachability (2). A mixture of fibers with very high and very low lignin contents will be more difficult to bleach than a uniform pulp. The variation will impact the amount of bleaching chemical required as well as the brightness and strength of the bleached pulp. Therefore, it is desirable to have a method to quantify fiber to fiber lignin content variation. Improved process control resulting from better monitoring of relevant fiber properties will also lead to lower bleaching costs, reduced environmental impact, and energy savings.
Our research group has discovered a method to optically determine lignin content of individual fibers. This method stains the fibers with the metachromatic fluorescent dye, Acridine Orange. It has been shown that at low lignin concentrations the stained fibers exhibit a green fluorescence, while at higher lignin content they exhibit a shift to red fluorescence. The ratio of red to green fluorescence correlates well with lignin content, when measured with a scanning microspectrofluorimeter (3,4).
Significant Progress to Date
With funding from the DOE, an on-line epi-illumination fluorescence macroscope has been designed to measure the lignin content of individual wood fibers at high rates of speed for a pulping process (see Figure 2). A xenon flashlamp provides an intense but short burst of light that effectively freezes the motion of the fibers in the flow cell. The first dichroic mirror reflects the blue portion of the light onto the flow cell. The green and red fluorescent light from the stained fibers passes through the first dichroic mirror. At this point, a second dichroic mirror reflects red light to the first digital camera, while passing the green light to the second camera. Both cameras are shuttered on in synchrony with the flash lamp to maximize the signal to background (stray) light.
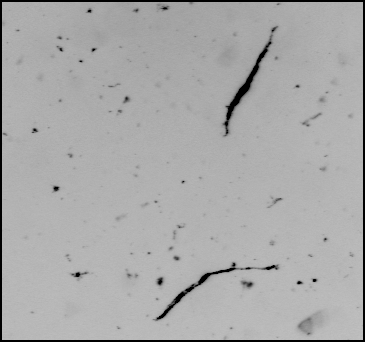
Once the red and green images have been captured in the frame buffers, a significant amount of post-processing is required. The algorithms must first perform scene segmentation, i.e., locate and isolate the (many) fibers in the image. This process is simplified if the contrast between the fibers and the surrounding solution is high, but this will not always be the case. Any background fluorescence from the solution must be taken into account. In addition, the software must be able to reject image artifacts, such as air bubbles and very small objects (see Figure 3). The software must be able to determine whether the detected object is a single fiber or a clump of fibers. Finally, the software must generate a histogram of relevant fiber properties, such as red/green fluorescence and fiber length, and also generate diagnostic reports to validate the results.
Up to this point we have developed an integrated software package that acquires the images, performs background subtraction and outlier rejection, reports the red/green fluorescence of individual fibers, and detects overlapped fibers. Our software is developed in the industry standard LabVIEW environment, which makes use of MMX processing on a Pentium II computer to dramatically shorten the analysis times of images to several per second.
We have investigated various fiber staining parameters. Originally, the stained fibers in the original staining solution were sent to the flow cell for detection. However, the background fluorescence of the staining solution interfered with the measurement of fiber fluorescence. One promising technique tested in the past quarter was to filter out the staining solution and reconstitute the stained fibers in pure water. The fibers appear to retain the stain for extended periods (weeks).
Proposed Research
The current DOE grant will support the completion of the development of this instrument and provide funding for us to test the instrument's use in kappa number determination. In particular we wish to optimize the staining protocol so that it applies to a wide variety of softwood species and mixtures of species. A proposal to carry out on-line kappa number measurements in pulp has been submitted to the NSF and EPA.
We have obtained further funding from CPAC to carry out studies of the application this instrument to other particle systems. Since the instrument can analyze for both morphological and chemical properties, it should be quite versatile. In particular, we are interested in measuring the growth status of cells (bacteria, yeast and mammalian) during a bioprocess. In addition, we believe that this instrument can be used to measure the flora and fauna in the surface waters of the open ocean. Funding will be sought for this application from the appropriate agencies, e. g. NOAA.
Literature Cited
1. Olson, J. A., Robertson, A. G., Finnigan, T. D. and Turner, R. R. H., "An Analyzer for Fibre Shape and Length," J. Pulp and Paper Science, 21 (11) J367-373.
2. Thomson, S.L. and Gustafson, R.R., "Effects of Kappa Variability on Pulp Properties," Proceedings of 1996 Tappi Pulping Conference, Nashville, p. 445 (1996).
3. Liu, Yue, "The Development of a Single Fiber Kappa Analyzer," Ph.D. Thesis, University of Washington, Seattle WA, 1998.
4. Liu, Y, and Gustafson, R.R. Callis, J., and McKean, W.T., Application of Single Fiber Fluorescence Staining to Assess Kappa Uniformity, Proceedings of 1998 Tappi Pulping Conference, Montreal Canada (1998), p1501.
Contact Us
|
Name |
Office |
Phone |
|
|
Professor James B. Callis |
Bagley 204A |
3-1208 |
callis@cpac.washington.edu |
|
Professor Richard L. Gustafson |
Bloedel 322 |
3-2790 |
pulp@u.washington.edu |
Figure 1. Flow-through Optical Cell Particles suspended in the sample stream are transported past the optical window. Auxiliary liquid sheathes the sample, narrowing the stream to align the particles and to bring them into optical focus.

Figure 2. Epi-Illuminated Optical System
In one possible configuration, blue light is reflected from the first dichroic mirror onto the flow cell, stimulating fluorescence. The red-shifted emission is collected and passed through the first dichroic mirror. The emitted light is then split by a second dichroic mirror, which reflects green light to one CCD and transmits red light to the second CCD.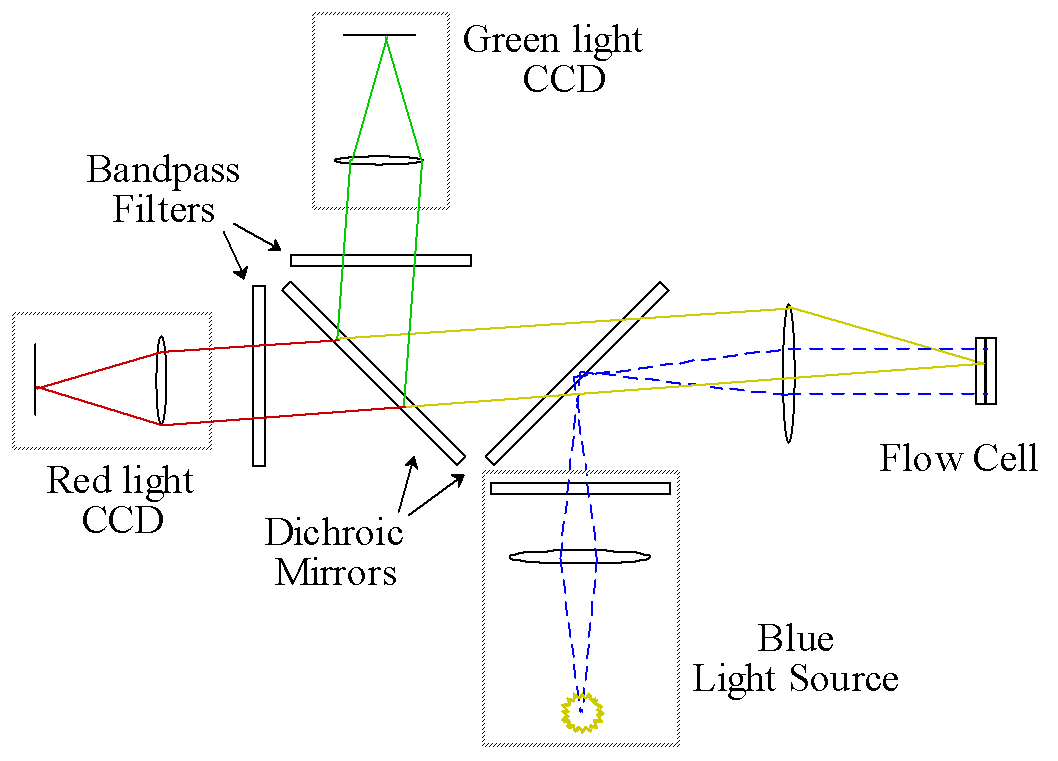
Figure 3. Raw and Processed Fiber Images (a) Shows an image of two fibers as they flow through the instrument. There are two fibers of interest, but there are numerous debris which contaminate the image. (b) The same image as (a) after correction for background fluorescence and removal of debris. The software can also distinguish between individual and aggregated particles.