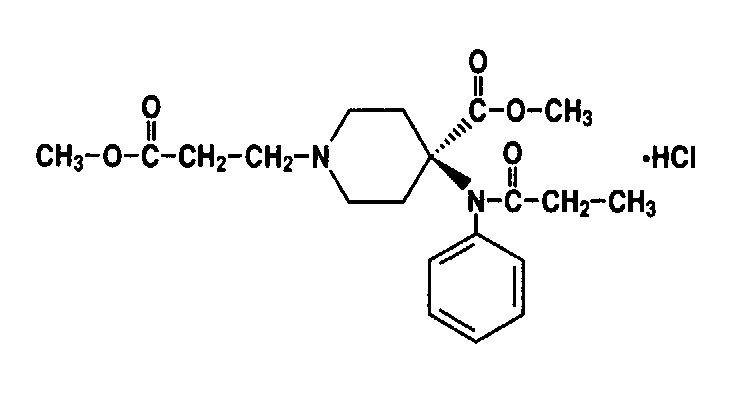
REMIFENTANIL
CLINICAL USE OF AN EVANESCENT OPIOID
JOHN BRAMHALL PhD MD
UNIVERSITY OF WASHINGTON, SEATTLE
OPIOIDS IN CLINICAL USE
Morphine and fentanyl are probably the two most widely used intra-operative
analgesics in current anesthetic practice. Fentanyl was patented
by Janssen in 1964 as a potent synthetic structural analog of
morphine; this was followed in the mid 1970s by sufentanil, and
in the late 1970s by alfentanil. These phenylpiperidines are structurally
closely related and differ primarily in their potency and in their
relative rates of redistribution and clearance. Loosely speaking,
the persistence of analgesic effect is: morphine>fentanyl>sufentanil>alfentanil
however, this simplistic sequence is confused by the phenomenon
of context sensitivity, a term used to describe the variation
in effective duration for a given dose of drug caused by the size
of the dose (or duration of administration). For example, the
effects of fentanyl are generally quite evanescent when given
as a single, small bolus; this is because the drug is rapidly
redistributed from the blood to other tissues. If, however, a
large dose is given, particularly over time as an infusion, then
redistribution routes are overwhelmed fentanyl then behaves as
a relatively persistent drug, longer acting in fact than morphine.
Sufentanil and alfentanil both display similar context sensitivity
but to a markedly lower extent because of variations in their
apparent volume of distribution and the differing rates at which
they are cleared, completely, from the body.
Multiple drugs are used to provide anesthesia, and volatile anesthetics are commonly combined with opioids. Several studies have demonstrated that small doses of opioid (i.e., within the analgesic range) result in a marked reduction in minimum alveolar concentration (MAC) of the volatile anesthetic that will prevent purposeful movement in 50% of patients at skin incision). However, further increases in opioid dose provide only a small additional reduction in MAC and a ceiling effect of the opioid is observed at a MAC value of the volatile anesthetic equal to its MAC awake. Recovery from anesthesia when an opioid is combined with a volatile anesthetic is dependent on the rate of decrease of both drugs to their respective concentrations that are associated with adequate spontaneous ventilation and awakening.
Volatile anesthetics can be administered in such a way as to maintain a relatively constant blood concentration of agent. This is facilitated by real-time assay of anesthetic levels in the patients blood by continuous sampling of exhaled vapors and subsequent fine calibration of inhaled dose. Intravenous agents are much harder to assay in real time so dosing is problematic if the goal is to attain defined, stable plasma concentrations of, say, opioid analgesic or hypnotic. Much effort has been expended in designing dosing algorithms which will establish these defined plasma concentrations of agent predictably in a variety of clinical settings. The phenomenon of context sensitivity, and multiple tissue sinks for drug redistribution complicates the calculations necessary to achieve this goal. Everything is so much simpler if a given drug is cleared, eliminated, rapidly and predictably from the body, rather than being shunted from compartment to compartment. Anesthesiologists have long recognized the need for a short-acting opioid with a predictable pharmacokinetic profile. This is the logical force leading to the commercial development of remifentanil.
GENERAL PROPERTIES OF REMIFENTANIL [3] [4]
Remifentanil is in the same structural family as fentanyl and
the other phenylpiperidines. It was brought to trials in 1991
and released to the North American market a couple of years ago.
It is synthesized by Upjohn and distributed by Glaxo Wellcome.
It is a novel, short-acting mu-receptor opioid agonist currently
in the late stages of world-wide distribution. A member of the
4-anilidopiperidine class, it is unique among the currently marketed
agents because of its ester structure. Remifentanil undergoes
widespread extrahepatic metabolism by blood and tissue nonspecific
esterases, resulting in an extremely rapid clearance of approximately
3 L/min. Like the other members of this class of drugs, remifentanil
is lipophilic and is widely distributed in body tissues with a
steady-state volume of distribution of approximately 30L. Because
of its unique metabolic pathway (among this group of drugs) and
rapid clearance, remifentanil represents a new pharmacokinetic
class of opioid. Unlike the other fentanyl congeners, termination
of the therapeutic effect of remifentanil mostly depends on metabolic
clearance rather than on redistribution. The context-sensitive
half- time is strikingly short for remifentanil, and this is perhaps
the most compelling evidence of the pharmacokinetic singularity
of the drug. The half- time is defined as the time necessary to
achieve a 50% decrease in blood (or plasma) concentration after
termination of a variable-length, continuous infusion targeted
to maintain a steady-state concentration, where the 'context'
is the duration of the infusion. Determined by computer simulation,
the context-sensitive half-time of remifentanil is approximately
3 minutes, and is, in fact, independent of infusion duration.
Pharmacodynamically, remifentanil is similar to the other fentanyl
congeners. The drug produces physiological changes consistent
with potent m-receptor agonist activity, including analgesia and
sedation. Its adverse effect profile (like that of the other drugs
of this class) includes ventilatory depression, nausea, vomiting,
muscular rigidity, bradycardia and pruritus. Because it does not
release histamine upon injection, remifentanil has fewer hemodynamic
adverse effects than morphine. The therapeutic potency of remifentanil
is somewhat less than that of fentanyl, with an effective concentration
(producing 50% of maximal effect, as measured by electroencephalography)
of approximately 10 to 20 mg/L. Onset of effect is very rapid
and is similar to that of alfentanil, which is reflected in a
t1/2eo (a parameter used to characterize the delay between peak
blood drug concentration and peak pharmacodynamic effect) of approximately
1 to 2 minutes.
The context-sensitive half-time, rather than the terminal elimination half-life, has been proposed as a more clinically relevant measure of decreasing drug concentration after a constant infusion of a given duration. The context-sensitive half-time is derived from computer modeling using known pharmacokinetic parameters. As noted above, the modeled context-sensitive half-time for a 3-h infusion of alfentanil is 50-55 min and is 3 min for remifentanil. The terminal elimination half-life is 111 min for alfentanil and 12-30 min for remifentanil. However, it is not obvious whether the modeled context-sensitive half-time simply reflects the time for a 50% decrease in drug concentration or of actual drug effect. In a recent study by Kapila and co-workers thirty volunteers received a 3-h infusion of remifentanil or alfentanil at equi-effective concentrations. Depression of minute ventilation to 7.5% ETCO2 was used as a measure of drug effect. Minute ventilation response was measured, and blood samples for drug concentration were taken during and after drug infusion. The recovery of minute ventilation (drug effect) and decrease in blood drug concentration was plotted, and the time for a 50% change was determined. The measured context-sensitive half-times were in close agreement with the context-sensitive half-times previously modeled for these drugs, confirming the value of the context-sensitive half-time in describing drug offset compared to the terminal elimination half-life.
INDUCTION [5]
Remifentanil is 15 times more potent than alfentanil, based on
the ED50 to achieve loss of response to a verbal command and 20
times more potent than alfentanil based on the EC50. Neither opioid
is suitable as a sole induction agent.
INTUBATION [6][7][8]
In a study of 80 healthy, premedicated patients with favorable
airway good or excellent intubation conditions (jaw relaxed, vocal
cords open, and fewer than two coughs in response to intubation)
were reliably attained 90 s after the administration of remifentanil
and propofol without use of neuromuscular relaxants. Remifentanil
1, 2, 3, or 4 mg/kg (groups I-IV, respectively) was infused intravenously
over 90 s. Sixty seconds after beginning the remifentanil infusion,
propofol 2 mg/kg was infused over 5 s. Ninety seconds after the
administration of propofol, laryngoscopy and tracheal intubation
were attempted and graded. Excellent intubating were observed
in 30%, 50%, 80%, 80% of patients in Groups I-IV, respectively.
Overall conditions at intubation were significantly better in
Groups III and IV compared with Groups I and II. The mean time
to resumption of spontaneous ventilation after induction was 5
min in all groups. No patient manifested clinically significant
muscle rigidity. The mean arterial pressure decreased 16%, 20%,
28%, 26% immediately before tracheal intubation in Groups I-IV,
respectively, no patient was treated for hypotension or bradycardia.
Note that the use of remifentanil i.v. bolus administration (in
contrast to controlled infusion) seems much more likely to induce
clinically significant bradycardia and/or muscle rigidity.
MAINTENANCE [9][10][11]
The interaction between opioids and volatile anesthetics is complex.
Defining this interaction provides a basis for more rational dosing
schemes when such combinations are used for anesthesia and allows
the anesthetic potency of remifentanil relative to other opioids
to be determined. Recently, two centers enrolled a total of 220
patients into a study in which MAC reduction of isoflurane by
remifentanil was determined. In this study, the MAC of isoflurane
alone was 1.3%. Remifentanil caused an exponential reduction in
the MAC of isoflurane with 1.37 ng/ml remifentanil a 77% reduction
and at 32 ng/ml a 91% reduction of isoflurane MAC. The MAC reduction
of isoflurane by remifentanil is similar to that produced by other
opioids. Although remifentanil was given at extremely high concentrations,
in the absence of isoflurane it did not provide adequate anesthesia.
A 50% isoflurane MAC reduction was produced by 1.37 ng/ml remifentanil
whole blood concentration (compared to previously published plasma
concentrations of fentanyl of 1.67 ng/ml or sufentanil of 0.14
ng/ml)., a similar study by Drover demonstrated that the remifentanil
blood concentration for which there is a 50% probability of adequate
anesthesia during abdominal surgery (Cb50) with 66% nitrous oxide
was 4.1 ng/ml in men and 7.5 ng/ml in women. The Cb50 values for
prostatectomy, nephrectomy, and other abdominal procedures were
3.8, 5.6, and 7.5 ng/ml, respectively. To put this data in a more
clinically useful form, a parallel study by Kovac’s group
demonstrated that a remifentanil infusion of 0.5 mg/kg/min is
as effective as an alfentanil infusion of 2 mg/kg/min in suppressing
intraoperative responses; doubling of the remifentanil infusion
to 0.5 mg/kg/min just before major surgical stress improves suppression
of responses and lowers general intraoperative use of remifentanil
without prolonging recovery times. Remifentanil certainly allows
faster awakening times than alfentanil, but pre-emptive administration
of postoperative analgesics is recommended to facilitate comfortable
discharge from the OR.
NEUROANESTHESIA [12][13][14][15]
Remifentanil appears to behave in a similar fashion to other agents
such as fentanyl and alfentanil and may offer significant advantages
for neurosurgical procedures in which prolonged anesthetic effects
can delay assessment of the patient. Remifentanil allows for early
neurologic evaluation without sacrificing the hemodynamic stability
of high-dose opioid techniques. Induction hemodynamics can be
controlled (remifentanil is 31 times more potent than alfentanil
for effects on MAP), ICP and cerebral perfusion pressure are relatively
stable for any given MAP and cerebrovascular CO2 reactivity is
maintained. When transcranial Doppler sonography was used to monitor
remifentanil-induced changes in cerebral perfusion it was found
that large doses of remifentanil reduced cerebral blood flow velocity
despite constant perfusion pressure. This may implicate a central
mechanism for cerebral hemodynamic effects of remifentanil.
In neurosurgical cases involving monitoring somatosensory evoked potentials, such as occurs with complex spinal surgery, variation of remifentanil infusion dosage provides an excellent method for responding to variable physiologic stressors (intermittent surgical stimulation) without compromising the electrophysiologic monitoring. Comparable variation of volatile agent concentration (e.g. isoflurane) invariably leads to changes in SSEP signal intensity that can be confusing when they occur at the same time as significant surgical manipulation of the cord structures.
PEDIATRICS [16]
Propofol-remifentanil-anesthesia (TIVA) was compared with a desflurane-N2O-anesthesia
(DN) with particular regard to the recovery of characteristics
in 50 children (4-11 years old) scheduled for ENT surgery. TIVA
was maintained with infusion of propofol and remifentanil, ventilation
was with oxygen in air. DN was maintained with desflurane in 50%
N20. The conclusion of the study was that TIVA with remifentanil
and propofol is a well-tolerated anesthesia method, with a lower
perioperative heart rate and less postoperative agitation compared
with a desflurane-N2O based anesthesia.
GERIATRICS [17][18]
The unique features of remifentanil are its rapid clearance and
binding kinetics, resulting in a rapid onset and offset of drug
effect. It is tempting to speculate that these characteristics
will make remifentanil an easy drug to titrate to effect, and
that clinicians will not need to consider patient covariates such
as advanced age when choosing a dosing regimen. By adjusting the
bolus and infusion doses, the anesthesiologist can hope to avoid
the peaks and valleys that might expose these patients to risk.
When the proper adjustment is made, the variability in remifentanil
pharmacokinetics should be considerably less than for any other
intravenous opioid. This ought to make remifentanil the most predictable
opioid for treatment of the elderly. However, when the pharmacokinetics
and pharmacodynamics of remifentanil were studied in complex simulation
exercises, and also in 65 healthy volunteers, using the electroencephalogram
(EEG) to measure the opioid effect, the infusion rate required
to maintain 50% EEG effect in a typical 80-yr old was found to
be approximately one third that required in a typical 20-yr old.
Failure to adjust the infusion rate for age resulted in a more
rapid onset of EEG effect and more profound steady-state EEG effect
in the simulated elderly population. Thus, because the EEG shows
increased brain sensitivity to opioids with increasing age, an
80-yr old person required approximately one half the bolus dose
of a 20-yr old of similar size and weight to reach the same peak
EEG effect. The simulations suggest that the time required for
a decrease in effect site concentrations will be more variable
in the elderly, and, as a result, elderly patients may occasionally
have a slower emergence from anesthesia than expected.
OBESITY [19]
The essential findings of a recent study by Egan et al. are that
remifentanil's pharmacokinetics are not appreciably different
in obese versus lean subjects and that remifentanil pharmacokinetic
parameters are therefore more closely related to LBM than to TBW.
Clinically this means that remifentanil dosing regimens should
be based on ideal body weight (or LBM) and not TBW. subjects and
that TBW-based dosing in obese patients can result in excessively
high remifentanil concentrations.
RENAL DISEASE [20][21]
An ester linkage renders remifentanil susceptible to rapid metabolism
by blood and tissue esterases; this hydrolysis produces a metabolite
with very weak opioid receptor activity that does not contribute
much to the opioid agonist effects of the parent drug. Thus it
was hypothesized that analgesic effectiveness would be unchanged
in cases of renal failure because remifentanil elimination itself
would be independent of renal function, and the principal metabolite
(GR90291), although eliminated renally, should cause little dependence
on renal function of opioid agonism. Studies have shown that this
is, indeed, the case. Patients with renal failure showed a marked
reduction in the elimination of GR90291; the half-life of the
metabolite increased from 1.5 h in the controls to more than 26
h in patients with renal failure. Thus the steady-state concentration
of GR90291 is likely to be more than 25 times higher in persons
with renal failure. However, there were no obvious differences
in opioid effects on minute ventilation in the controls and in
patients with renal failure; the pharmacokinetics and pharmacodynamics
of remifentanil were not altered in patients with renal disease,
even though the elimination of its principal metabolite was markedly
reduced. Based on simulations, the concentration of GR90291 at
the end of a 12-h remifentanil infusion of 2 mg/kg/min is not
likely to produce significant opioid effects.
HEPATIC DISEASE [22]
By similar logic (above) clearance should also be relatively unaffected
by changes in hepatic function. Ten volunteers with chronic, stable,
severe hepatic disease and awaiting liver transplantation and
ten matched controls were studied. Each subject was given a 4-h
infusion of remifentanil; the first five pairs received 0.0125
mg/kg/min for 1 h followed by 0.025 mg/kg/min for 3 h; the second
five pairs received double these infusion rates. There were no
differences in any of the pharmacokinetic parameters for remifentanil
or GR90291 between the two groups. However, the subjects with
liver disease were noted to be more sensitive to the ventilatory
depressant effects of remifentanil, a finding of uncertain clinical
significance, considering the extremely short duration of action
of the drug.
CARDIAC DISEASE [21][23]
Opioids decrease the sympathetic and somatic responses to noxious
stimulation and can be given in high doses without negative inotropic
effects, even in patients with impaired cardiac function. With
currently available opioids, precise titration of dose to effect
is often quite difficult, and high doses result in drug accumulation
and prolonged respiratory depression. Remifentanil has a rapid
onset, short latency to peak effect, and a very short duration
of action when withheld; it should be ideal for many cardiac cases.
To assess the effects of cardiopulmonary bypass on remifentanil
pharmacokinetics, sixteen patients undergoing coronary revascularization
requiring cardiopulmonary bypass (CPB) received remifentanil (2
or 5 mg/kg/min) by infusion over 1 min after sternotomy but before
commencing cardiopulmonary bypass, during hypothermic CPB and
during CPB after rewarming. Hypothermic CPB reduced the clearance
of remifentanil by an average of 20%, and this was attributed
to the effect of temperature on blood and tissue esterase activity.
Reductions in arterial pressure occurred with administration of
both doses during normothermia only.
AMBULATORY SURGERY [24] [25] [26]
Patients treated with regional anesthesia often require concomitant
medication for comfort and sedation propofol is widely used for
this purpose. Because remifentanil seems to exhibit, at low doses,
distinct sedative properties it may be useful for supplementation
of regional anesthesia. Several recent studies have attempted
to compare remifentanil to a propofol-based sedation technique
for monitored anesthesia care. In one, 44 patients were enrolled
in a recent study and received intravenous midazolam 2 mg, followed
by a continuous infusion of either propofol 75 mg/kg/min, or remifentanil
0.1 mg/kg/min (which was subsequently titrated to maintain optimal
patient comfort without respiratory depression). Surgical-related
pain was treated by injecting additional local anesthetic solution
and "rescue" boluses of fentanyl 25 mg IV. Remifentanil
provided comparable intraoperative conditions and patient comfort
at a lower sedation level compared with propofol. However use
of remifentanil resulted in greater respiratory depression compared
with propofol, with decreases in the remifentanil infusion rate
required by 41% of patients because of a slow respiratory rate
(<8 breaths/min) and/or oxygen desaturation measured by pulse
oximetry (SpO2 <90%). Median times to ambulation and to being
judged "fit for discharge" were significantly shorter
following propofol (40 and 47 minutes, respectively) compared
with remifentanil (52 and 58 minutes, respectively).
In another, similar, study of 107 ASA I-III adult patients who underwent orthopedic or urogenital surgery with axillary, ankle, or spinal block. Patients were randomized to receive either an infusion of remifentanil 0.2 mg/kg/min or propofol 100 mg/kg/min 5 minutes before nerve block placement; the infusions were decreased by 50% on block completion, increased by 50% for patient discomfort, and decreased by 50% for hypoventilation (<8 breaths/min) or hemodynamic instability. At the doses studied, remifentanil was more effective than propofol in minimizing pain without producing excessive sedation, however it was associated with more transient respiratory depression and troublesome short-term nausea. The recommendation was made that the initial remifentanil rate should be 0.1 mg/kg/min (50% lower than the study's initial rate) and should be further decreased in the elderly to minimize adverse effects.
In another open, prospective trial, 28 patients were randomly
allocated to receive continuous infusions of remifentanil (6 mg/kg/min)
or propofol (180 mg/kg/min) for sedation during spinal or axillary
regional anesthesia. Infusion rates were titrated to maintain
a sedation level > or = 2 as assessed with the Observer's Assessment
of Alertness Scale.
Return to alertness occurred after 10 (+/- 6 min) in the remifentanil
group and after 16 (+/- 15 min) in the propofol group. Similar
incidences of hypotension, bradycardia, and nausea and vomiting
were found in both groups, but intraoperative respiratory depression
and nausea were more prominent in the remifentanil group. The
conclusion of the authors was that, when titrated to the same
sedation level, remifentanil provided a smoother hemodynamic profile
than propofol during regional anesthesia, but that the frequent
occurrence of remifentanil-induced respiratory depression requires
very cautious administration of this agent.
OBSTETRICS [27]
Kan et al. evaluated the placental transfer of remifentanil and
the neonatal effects when it is administered as an intravenous
infusion. Nineteen parturients undergoing nonemergent cesarean
section with epidural anesthesia received 0.1 mg/kg/min remifentanil
intravenously, which was continued until skin closure. Maternal
arterial (MA), umbilical arterial (UA), and umbilical venous (UV)
blood samples were obtained at delivery for analysis of drug concentrations
of remifentanil, its metabolite, and blood gases. The means and
SDs of UV:MA and UA:UV ratios for remifentanil were 0.88+/-0.78
and 0.29+/-0.07, respectively. Mean clearance was 93 ml/min/kg.
The mean UV:MA and UA:MV ratios for GR90291 (remifentanil acid)
were 0.56+/-0.29 and 1.23+/-0.89, respectively. The mean MA (remifentanil
acid):MA (remifentanil) ratio was 2.92+/-3.65. There were no adverse
effects on the neonates, but there was a sedative effect and respiratory
depressant effect on the mothers. Thus, remifentanil certainly
crosses the placenta but appears to be rapidly metabolized, redistributed,
or both. With well-controlled infusions maternal sedation and
respiratory changes occur, but without apparent adverse neonatal
or maternal effects.
TIVA [28][29]
"Smart" infusion pumps for the administration of propofol
by target controlled infusion are now commercially available and
are becoming more widely used. Among currently available analgesic
drugs, alfentanil and remifentanil are considered to be the most
suitable for administration by target controlled infusion, but
commercial systems for these agents are not yet available. The
goal is to deliver regulated infusion of drug to attain constant
steady-state plasma concentration without necessitating regular
assay of blood samples.
The whole approach is quite complex when the drug in question
undergoes multiple transformations and/or redistribution. In addition,
constant plasma levels may not equate to constant clinical effectiveness;
for example tolerance to opioid agonists can be profound and may
develop very rapidly. Studies in experimental animals have demonstrated
a rapidly developing acute tolerance to the analgesic effect of
opioids administered by continuous i.v. infusion. The analgesic
effect of remifentanil, infused at a constant rate of 0.1 mg/kg/min
for 4 h, was evaluated by measuring pain tolerance with thermal
(2 degrees C water) and mechanical (pressure) noxious stimulations
in 13 paid volunteers. The constant-rate infusion of remifentanil
resulted in a threefold increase in pain tolerance with both tests.
After reaching its maximum in 60-90 min, the analgesic effect
of remifentanil began to decline despite the constant-rate infusion,
and after 3 h of infusion, it was only one fourth of the peak
value. Leading to the conclusion that the development of tolerance
must also be included in the calculations for target-controlled
infusions.
CAVEAT [21][30][31]
Possible disadvantages of the drug in certain situations include:
the need to mix the lyophilized drug with a diluent, difficulties
experienced with bolus administration, and resulting need for
administration as a continuous infusion, risk of rapid loss of
analgesic and effects if the infusion is interrupted accidentally,
and difficulty in judging the dose of another, longer lasting
opioid that will be required to control postoperative pain without
producing excessive ventilatory depression. This transition from
remifentanil intraoperative anesthesia to postoperative analgesia
must be planned carefully because of its very short duration of
action (3-10 min) of. Remifentanil is also likely to be more expensive
than other opioids, but its use might reduce overall costs if
prompt recovery from its effects results in shorter stays in the
operating room and recovery units.
COST
Current costs of synthetic opioids as determined by the University
of Washington pharmacies are listed below for general comparative
purposes (US$):
FENTANYL (250 mg) 0.42
SUFENTANIL (250 mg) 23.77
ALFENTANIL (2,500 mg) 15.95
REMIFENTANIL (5,000 mg) 61.03
Again, for comparative purposes, a typical opioid infusion during a 2-4 hour general surgical case might be 2 mg/kg/h for fentanyl, 0.5 mg/kg/h for sufentanil, and 15 mg/kg/h for remifentanil.
CONCLUSION [32] [21]
Remifentanil is a selective m opioid receptor agonist, of higher
potency than alfentanil, but with pharmacological effects that
essentially parallel those of alfentanil and other opioids in
this class. Unlike these other opioids, however, remifentanil
is rapidly hydrolyzed by nonspecific plasma and tissue esterases:
this imparts brevity of action, precise and easily titrated effects
(attributable to rapid onset and offset kinetics), non- cumulative
opioid effects and rapid recovery after cessation of administration
(attributable to rapid clearance). The onset of action of remifentanil
is similar to that of alfentanil, although its offset is considerably
more rapid and independent of the duration of infusion.
The context-sensitive half-life remains very short (3 to 4 minutes),
independent of the duration of infusion. These characteristics
facilitate titration of dose to effect and also allow the use
of very high doses (ED99) without fear of prolonging recovery.
The unique pharmacokinetic profile of remifentanil facilitates 'real time' management of intraoperative stress, as well as provision of optimal intraoperative analgesia without compromising recovery for a variety of surgical procedures. It is tempting to speculate that these characteristics will make remifentanil an easy drug to titrate, and also that clinicians will have less need to consider patient covariates such as extremes of age or size when choosing a dosing regimen. However, the rapid onset of drug effect may be accompanied by rapid onset of adverse events such as apnea and muscle rigidity. The rapid offset of drug effect can result in patients who are in severe pain at a time when the anesthesiologist is ill equipped to deal the problem - for example when the patient is in transit to the recovery room.
CITATIONS
1.Hughes, M.A., Glass P.S., Jacobs J.R., Context-sensitive half-time
in multicompartment pharmacokinetic models for intravenous anesthetic
drugs. Anesthesiology, 1992. 76: p. 334-.
2.Glass, P.S., et al., Drug interactions: volatile anesthetics
and opioids. J Clin Anesth, 1997. 9(6 Suppl): p. 18S-22S.
3.Egan, T.D., Remifentanil pharmacokinetics and pharmacodynamics.
A preliminary appraisal. Clin Pharmacokinet, 1995. 29(2): p. 80-94.
4.Kapila, A., et al., Measured context-sensitive half-times of
remifentanil and alfentanil [see comments]. Anesthesiology, 1995.
83(5): p. 968-75.
5.Jhaveri, R., et al., Dose comparison of remifentanil and alfentanil
for loss of consciousness. Anesthesiology, 1997. 87(2): p. 253-9.
6.Stevens, J.B. and L. Wheatley, Tracheal intubation in ambulatory
surgery patients: using remifentanil and propofol without muscle
relaxants. Anesth Analg, 1998. 86(1): p. 45-9.
7.Thompson, J.P., et al., Effect of remifentanil on the haemodynamic
response to orotracheal intubation. Br J Anaesth, 1998. 80(4):
p. 467-9.
8.Hogue, C.W., Jr., et al., A multicenter evaluation of total
intravenous anesthesia with remifentanil and propofol for elective
inpatient surgery. Anesth Analg, 1996. 83(2): p. 279-85.
9.Lang, E., et al., Reduction of isoflurane minimal alveolar concentration
by remifentanil. Anesthesiology, 1996. 85(4): p. 721-8.
10.Drover, D.R. and H.J. Lemmens, Population pharmacodynamics
and pharmacokinetics of remifentanil as a supplement to nitrous
oxide anesthesia for elective abdominal surgery [In Process Citation].
Anesthesiology, 1998. 89(4): p. 869-77.
11.Kovac, A.L., et al., Remifentanil versus alfentanil in a balanced
anesthetic technique for total abdominal hysterectomy. J Clin
Anesth, 1997. 9(7): p. 532-41.
12.Warner, D.S., et al., Intracranial pressure and hemodynamic
effects of remifentanil versus alfentanil in patients undergoing
supratentorial craniotomy. Anesth Analg, 1996. 83(2): p. 348-53.
13.Guy, J., et al., Comparison of remifentanil and fentanyl in
patients undergoing craniotomy for supratentorial space-occupying
lesions. Anesthesiology, 1997. 86(3): p. 514-24.
14.Paris, A., et al., The effect of remifentanil on cerebral blood
flow velocity. Anesth Analg, 1998. 87(3): p. 569-73.
15.Ostapkovich, N.D., et al., Cerebral blood flow and CO2 reactivity
is similar during remifentanil/N2O and fentanyl/N2O anesthesia.
Anesthesiology, 1998. 89(2): p. 358-63.
16.Grundmann, U., et al., Total intravenous anaesthesia with propofol
and remifentanil in paediatric patients: a comparison with a desflurane-nitrous
oxide inhalation anaesthesia. Acta Anaesthesiol Scand, 1998. 42(7):
p. 845-50.
17.Shafer, S.L., The role of newer opioids in geriatric anesthesia.
Acta Anaesthesiol Belg, 1998. 49(2): p. 91-103.
18.Minto, C.F., T.W. Schnider, and S.L. Shafer, Pharmacokinetics
and pharmacodynamics of remifentanil. II. Model application. Anesthesiology,
1997. 86(1): p. 24-33.
19.Egan, T.D., et al., Remifentanil pharmacokinetics in obese
versus lean patients [see comments]. Anesthesiology, 1998. 89(3):
p. 562-73.
20.Hoke, J.F., et al., Pharmacokinetics and pharmacodynamics of
remifentanil in persons with renal failure compared with healthy
volunteers. Anesthesiology, 1997. 87(3): p. 533-41.
21.Michelsen, L.G. and C.C. Hug, Jr., The pharmacokinetics of
remifentanil. J Clin Anesth, 1996. 8(8): p. 679-82.
22.Dershwitz, M., et al., Pharmacokinetics and pharmacodynamics
of remifentanil in volunteer subjects with severe liver disease.
Anesthesiology, 1996. 84(4): p. 812-20.
23.Russell, D., et al., Effect of temperature and cardiopulmonary
bypass on the pharmacokinetics of remifentanil. Br J Anaesth,
1997. 79(4): p. 456-9.
24.Smith, I., M.N. Avramov, and P.F. White, A comparison of propofol
and remifentanil during monitored anesthesia care. J Clin Anesth,
1997. 9(2): p. 148-54.
25.Mingus, M.L., et al., Remifentanil versus propofol as adjuncts
to regional anesthesia. Remifentanil 3010 Study Group. J Clin
Anesth, 1998. 10(1): p. 46-53.
26.Lauwers, M.H., C. Vanlersberghe, and F. Camu, Comparison of
remifentanil and propofol infusions for sedation during regional
anesthesia. Reg Anesth Pain Med, 1998. 23(1): p. 64-70.
27.Kan, R.E., et al., Intravenous remifentanil: placental transfer,
maternal and neonatal effects. Anesthesiology, 1998. 88(6): p.
1467-74.
28.Milne, S.E. and G.N. Kenny, Future applications for TCI systems.
Anaesthesia, 1998. 53 Suppl 1: p. 56-60.
29.Vinik, H.R. and I. Kissin, Rapid development of tolerance to
analgesia during remifentanil infusion in humans. Anesth Analg,
1998. 86(6): p. 1307-11.
30.Yarmush, J., et al., A comparison of remifentanil and morphine
sulfate for acute postoperative analgesia after total intravenous
anesthesia with remifentanil and propofol [see comments]. Anesthesiology,
1997. 87(2): p. 235-43.
31.Schuttler, J., et al., A comparison of remifentanil and alfentanil
in patients undergoing major abdominal surgery [see comments].
Anaesthesia, 1997. 52(4): p. 307-17.
32.Patel, S.S. and C.M. Spencer, Remifentanil. Drugs, 1996. 52(3):
p. 417-27; discussion 428.